Abstract
Transthyretin amyloidosis with polyneuropathy (ATTR-PN), a rare and progressive hereditary disorder, results from mutations in the gene coding for the transthyretin (TTR) protein that destabilize the protein’s tetrameric structure. In over 40 countries worldwide, tafamidis (Vyndaqel®) is approved for the treatment of TTR amyloidosis in adults with stage 1 symptomatic polyneuropathy, to delay peripheral neurological impairment. Tafamidis is administered orally once daily, as a soft capsule. Evidence from clinical studies, including an 18-month placebo-controlled trial and subsequent long-term, open-label extension studies (providing data from ≤ 6 years of treatment), indicate that tafamidis slowed deterioration of neurological function and maintained health-related quality of life in patients with early-stage ATTR-PN and the Val30Met mutation. TTR tetramers were stabilized in nearly all patients, and nutritional status was generally maintained or improved. Similar benefit was seen with tafamidis over 12 months in a noncomparative trial in patients with non-Val30Met ATTR-PN, although disease progression in this population (which was older and had had ATTR-PN for longer than Val30Met patients) became more notable with continued therapy in an extension study. Data for up to 10 years from large registry and referral centre studies support the long-term effectiveness and safety of tafamidis in delaying disease progression and conferring survival benefits in patients with stage 1 ATTR-PN. Tafamidis was generally well tolerated, with no new safety signals detected during the long-term trial or real-world experience. Thus, based on up to 10 years’ experience, tafamidis continues to be a valuable option in the treatment of early-stage ATTR-PN.


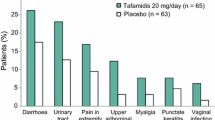
(adapted from Scott [11])
Similar content being viewed by others
References
Ando Y, Coelho T, Berk JL, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31.
Hou X, Aguilar M-I, Small DH. Transthyretin and familial amyloidotic polyneuropathy: recent progress in understanding the molecular mechanism of neurodegeneration. FEBS J. 2007;274(7):1637–50.
Sekijima Y, Ueda M, Koike H, et al. Diagnosis and management of transthyretin familial amyloid polyneuropathy in Japan: red-flag symptom clusters and treatment algorithm. Orphanet J Rare Dis. 2018;13(1):6.
Schmidt HH, Waddington-Cruz M, Botteman MF, et al. Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2018;57(5):829–37.
Adams D, Suhr OB, Hund E, et al. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol. 2016;29(Suppl 1):S14–26.
Hawkins PN, Ando Y, Dispenzeri A, et al. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–38.
Ericzon BG, Wilczek HE, Larsson M, et al. Liver transplantation for hereditary transthyretin amyloidosis: after 20 years still the best therapeutic alternative? Transplantation. 2015;99(9):1847–54.
Sekijima Y. Recent progress in the understanding and treatment of transthyretin amyloidosis. J Clin Pharm Ther. 2014;39(3):225–33.
Plante-Bordenave V. Transthyretin familial amyloid polyneuropathy: an update. J Neurol. 2018;265:976–83.
Coelho T, Merlini G, Bulawa CE, et al. Mechanism of action and clinical application of tafamidis in hereditary transthyretin amyloidosis. Neurol Ther. 2016;5(1):1–25.
Scott LJ. Tafamidis: a review of its use in familial amyloid polyneuropathy. Drugs. 2014;74(12):1371–8.
Bulawa CE, Connelly S, DeVit M, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. 2012;109(24):9629–34.
European Medicines Agency. Vyndaqel (tafamidis) 20 mg soft capsules: summary of product characteristics. 2016. http://www.ema.europa.eu/. Accessed 2 May 2019.
Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–92.
Coelho T, Maia LF, Martins da Silva A, et al. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260(11):2802–14.
Barroso FA, Judge DP, Ebede B, et al. Long-term safety and efficacy of tafamidis for the treatment of hereditary transthyretin amyloid polyneuropathy: results up to 6 years. Amyloid. 2017;24(3):194–204.
Ando Y, Sekijima Y, Obayashi K, et al. Effects of tafamidis treatment on transthyretin (TTR) stabilization, efficacy, and safety in Japanese patients with familial amyloid polyneuropathy (TTR-FAP) with Val30Met and non-Val30Met: a phase III, open-label study. J Neurol Sci. 2016;362:266–71.
Merlini G, Planté-Bordeneuve V, Judge DP, et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis. J Cardiovasc Transl Res. 2013;6(6):1011–20.
Amass L, Li H, Gundapaneni B, et al. Influence of baseline neurologic severity on disease progression and the associated disease-modifying effects of tafamidis in patients with transthyretin amyloid polyneuropathy. Orphanet J Rare Dis. 2018;13:225.
Gundapaneni BK, Sultan MB, Keohane DJ, et al. Tafamidis delays neurological progression comparably across Val30Met and non-Val30Met genotypes in transthyretin familial amyloid polyneuropathy. Eur J Neurol. 2018;25(3):464–8.
Keohane D, Schwartz J, Gundapaneni B, et al. Tafamidis delays disease progression in patients with early stage transthyretin familial amyloid polyneuropathy: additional supportive analyses from the pivotal trial. Amyloid. 2017;24(1):30–6.
Waddington Cruz M, Amass L, Keohane D, et al. Early intervention with tafamidis provides long-term (5.5-year) delay of neurologic progression in transthyretin hereditary amyloid polyneuropathy. Amyloid. 2016;23(3):178–83.
Coelho T, Inês M, Conceição I, et al. Natural history and survival in stage I Val30Met transthyretin familial amyloid polyneuropathy. Neurology. 2018;91(21):e1999–2009.
Cortese A, Vita G, Luigetti M, et al. Monitoring effectiveness and safety of tafamidis in transthyretin amyloidosis in Italy: a longitudinal multicenter study in a non-endemic area. J Neurol. 2016;263(5):916–24.
Mundayat R, Stewart M, Alvir J, et al. Positive effectiveness of tafamidis in delaying disease progression in transthyretin familial amyloid polyneuropathy up to 2 years: an analysis from the Transthyretin Amyloidosis Outcomes Survey (THAOS). Neurol Ther. 2018;7(1):87–101.
Plante-Bordeneuve V, Gorram F, Salhi H, et al. Long-term treatment of transthyretin familial amyloid polyneuropathy with tafamidis: a clinical and neurophysiological study. J Neurol. 2017;264(2):268–76.
Sousa S, Valdrez K, Fonseca I, et al. Prediction of nerve conduction studies outcomes in patients with familial amyloidotic polyneuropathy receiving tafamidis therapy. J Neuromuscul Dis. 2016;3(Suppl 1):S77–8.
European Medicines Agency. Tafamidis: CHMP assessment report. 2011. http://www.ema.europa.eu/. Accessed 2 May 2019.
Damy T, Judge DP, Kristen AV, et al. Cardiac findings and events observed in an open-label clinical trial of tafamidis in patients with non-Val30Met and non-Val122Ile hereditary transthyretin amyloidosis. J Cardiovasc Transl Res. 2015;8(2):117–27.
Pfizer Inc. Vyndaqel® capsules 20 mg: Japanese prescribing information. 2016. http://www.pmda.go.jp/. Accessed 2 May 2019.
McKeage K, Lyseng-Williamson KA, Scott LJ. Tafamidis in transthyretin amyloidosis: a guide to its use in delaying peripheral neurological impairment in patients with stage 1 polyneuropathy. Drug Ther Perspect. 2017;33(2):47–53.
Pinto MV, Barreira AA, Bulle AS, et al. Brazilian consensus for diagnosis, management and treatment of transthyretin familial amyloid polyneuropathy. Arq Neuropsiquiatr. 2018;76(9):609–21.
Keam SJ. Inotersen: first global approval. Drugs. 2018;78:1–6.
Hoy S. Patisiran: first global approval. Drugs. 2018;78:1625–31.
Alnylam Pharmaceuticals Inc. ONPATTRO (patisiran) lipid complex injection, for intravenous use. 2018. http://www.alnylam.com/. Accessed 2 May 2019.
Ionis Pharmaceuticals Inc. TEGSEDI (inotersen) injection, for subcutaneous use. 2018. http://tegsedi.com/. Accessed 2 May 2019.
Adams D, Théaudin M, Cauquil C, et al. FAP neuropathy and emerging treatments. Curr Neurol Neurosci Rep. 2014;14(3):435.
Mariani L-L, Lozeron P, Théaudin M, et al. Genotype-phenotype correlation and course of transthyretin familial amyoid polyneuropathies in France. Ann Neurol. 2015;78(6):901–16.
Lozeron P, Theaudin M, Mincheva Z, et al. Effect on disability and safety of tafamidis in late onset of Met30 transthyretin familial amyloid polyneuropathy. Eur J Neurol. 2013;20(12):1539–45.
Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;27:1007–16.
Vita G, Vita GL, Stancanelli C, et al. Genetic neuromuscular disorders: living the era of a therapeutic revolution. Part 1: peripheral neuropathies. Neurol Sci. 2019;40(4):661–9.
Plante-Bordeneuve V, Lin H, Gollob J, et al. An indirect treatment comparison of the efficacy of patisiran and tafamidis for the treatment of hereditary transthyretin-mediated amyloidosis with polyneuropathy. Expert Opin Pharmacother. 2018;20(4):473–81.
Klamerus KJ, Watsky E, Moller R, et al. The effect of tafamidis on the QTc interval in healthy subjects. Br J Clin Pharmacol. 2015;79(6):918–25.
Suhr OB, Conceicao IM, Karayal ON, et al. Post hoc analysis of nutritional status in patients with transthyretin familial amyloid polyneuropathy: impact of tafamidis. Neurol Ther. 2014;3(2):101–12.
Acknowledgements
During the peer review process, the manufacturer of tafamidis was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Yvette Lamb and Emma Deeks are salaried employees of Adis/Springer, responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by:F. Barroso, Department of Neurology, Institute for Neurological Research Raul Carrea (FLENI), Buenos Aires, Argentina; M. Waddington Cruz, National Amyloidosis Referral Center (CEPARM), University Hospital, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Rights and permissions
About this article
Cite this article
Lamb, Y.N., Deeks, E.D. Tafamidis: A Review in Transthyretin Amyloidosis with Polyneuropathy. Drugs 79, 863–874 (2019). https://doi.org/10.1007/s40265-019-01129-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01129-6