Abstract
Positive experience with inhaled antibiotics in pulmonary infections of patients with cystic fibrosis has paved the way for their utilization in mechanically ventilated, critically ill patients with lower respiratory tract infections. A successful antibiotic delivery depends upon the size of the generated particle and the elimination of drug impaction in the large airways and the ventilator circuit. Generated droplet size is mainly affected by the type of the nebulizer employed. Currently, jet, ultrasonic, and vibrating mesh nebulizers are marketed; the latter can deliver optimal antibiotic particle size. Promising novel drug-device combinations are able to release drug concentrations of 25- to 300-fold the minimum inhibitory concentration of the targeted pathogens into the pulmonary alveoli. The most important practical steps of nebulization include pre-assessment and preparation of the patient (suctioning, sedation, possible bronchodilation, adjustment of necessary ventilator settings); adherence to the procedure (drug preparation, avoidance of unnecessary tubing connections, interruption of heated humidification, removal of heat-moisture exchanger); inspection of the procedure (check for residual in drug chamber, change of expiratory filter, return sedation, and ventilator settings to previous status); and surveillance of the patient for adverse events (close monitoring of the patient and particularly of peak airway pressure and bronchoconstriction). Practical aspects of nebulization are very important to ensure optimal drug delivery and safe procedure for the patient. Therefore, the development of an operational checklist is a priority for every department adopting this modality.
Similar content being viewed by others
Delivery of inhaled antibiotics in mechanically ventilated patients is a modality with increasing interest and utilization among intensivists, without well-recognized standard procedures. |
The type of nebulizer used is the major determinant of all practical issues. |
The most important practical steps of nebulization include pre-assessment and preparation of the patient; adherence to the procedure (drug preparation, ventilator settings, and circuit adjustments); and monitoring of the procedure for completeness and safety. |
1 Introduction
Inhaled antibiotics were introduced in the treatment of acute and chronic bacterial infections of the airways in the 1940s, with the first attempts of aerosolized neomycin, polymyxin, and penicillin G [1, 2]. However, they failed to gain acceptance owing to reports of superinfections with resistant strains to polymyxin B in 1975, probably attributable to inadequate delivery systems [3, 4]. In the late 1990s, the interest for inhaled antibiotics and particularly tobramycin in the management of patients with cystic fibrosis (CF) and chronic Pseudomonas aeruginosa colonization was rekindled [5]. Experience in this particular group of patients was followed by an impressive evolution of drug-delivery devices [6]. Recently, the emergence of the ESKAPE group of multidrug-resistant (MDR) pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter spp.) has significantly challenged the treatment of patients in intensive care units with ventilator-associated pneumonia (VAP) and other respiratory infections [7, 8].
As a consequence, similar epidemiological features with patients with CF have prompted the use of inhaled antibiotics in mechanically ventilated (MV) patients, despite scarce clinical evidence [9]. Nebulized antibiotics have been administered in a wide array of indications in MV patients, mainly in the treatment of VAP and ventilator-associated tracheobronchitis [10, 11]. The clinical benefit is yet to be evaluated because studies are mostly observational and heterogeneous, employing either an intravenous (i.v.) plus inhaled form of the same antibiotic, or an inhaled approach adjunctive to other i.v. antibiotics, and even an inhaled-only approach [12,13,14]. Two recent surveys have explored the real-life use of inhaled antibiotics by intensive care physicians [11, 15, 16]. No standardized technique, dosage, and delivery system was employed, and indications and antibiotics used were variable. Both surveys elucidated prescribers’ uncertainty about practical aspects of nebulization and the need for the development of guidance on the use of inhaled antibiotics for MV patients. The aim of this review is to summarize published evidence relating to the practical aspects of nebulization and help to optimize the use of this new modality of antibiotic delivery in MV patients. Assessment of the clinical efficacy of inhaled antibiotics in MV patients is beyond the scope of the article and is detailed in other recent reviews [10, 17, 18] and systematic reviews [12,13,14]. The term ‘inhaled antibiotics’ was adopted in this article to describe using antibiotics via a nebulizer; statements made should not be extrapolated to other types of delivery devices or to inhaled antibiotics in general.
2 Rationale and Description of the Technique
The bronchial mucosa, epithelial lining fluid (i.e., interstitial/extracellular space), and alveolar macrophages (i.e., intracellular space) offer interfaces to evaluate drug disposition into the various compartments of the lung. The lung is a difficult organ; many systemically administered antibiotics achieve lower concentrations at the target site for conventional extracellular pathogens (i.e., interstitial space) compared with the blood concentrations [19]. This may be attributed to their inability to cross the alveolar-capillary barrier (vancomycin, colistin, aminoglycosides), or achieve predictable concentrations in inflamed and consolidated lung areas [17, 19, 20]. Furthermore, in MV patients, altered antibiotic pharmacokinetics has been recently recognized as an important factor compromising optimal drug penetration [21, 22].
Moreover, the inability to achieve concentrations above the minimum inhibitory concentration (MIC) of the targeted pathogen predisposes to an increased probability of clinical failure; overcoming mutant prevention concentration might prove to be even more challenging [23,24,25]. In addition to this, the presence of the endotracheal tube, being responsible for biofilm formation, makes eradication of bacteria extremely difficult [26]. The ESKAPE pathogens, usually exhibiting a MDR or even pan-drug-resistant profile, impose an additional challenge in the treatment of respiratory infections in MV patients. Importantly, as MDR and pan-drug resistant pathogens have been associated with worse clinical outcomes compared with susceptible counterparts, epidemiological data underline the need for early appropriate treatment to ensure reduction in mortality [7, 27, 28].
In this epidemiologic milieu, porcine experimental studies have demonstrated that inhaled antibiotics achieved 30- to 200-fold higher lung tissue antibiotic concentrations and higher bactericidal activity when compared with similar amounts of intravenously administered antibiotics [6]. Other experimental studies showed that aminoglycosides, despite poor penetration in the lung parenchyma after i.v. administration, when given in an inhaled form, diffuse in the systemic circulation through inflamed lung tissues but maintain non-toxic serum levels even when administered in high doses [29,30,31,32,33,34]. In contrast, colistin is almost undetectable in lung tissues after i.v. administration and poorly absorbed in the systemic circulation when given by inhalation, even in the presence of lung inflammation [35].
Based on the above-mentioned challenges, increased interest in the use of inhaled antibiotics in MV patients is an attempt to: (1) overcome the generally reduced penetration of antimicrobials into the interstitial space of the lung; (2) treat pulmonary infections by pathogens resistant according to the established breakpoints based on serum pharmacokinetics; (3) prevent the emergence of resistant pathogens; (4) avoid systemic toxicity caused by i.v. administration of boosted doses; and ultimately (5) impact positively on patients’ outcomes [10, 17].
An efficient delivery of antibiotics in the lung parenchyma requires an optimal aerosol generator (the nebulizer), a carefully selected and properly prepared antibiotic solution, and finally a wisely selected patient, who has to be properly prepared along with all the ventilator and circuit adjustments [10]. Efficacy of the procedure depends largely on the size of the generated aerosol particle, the synchronization of the patient with the nebulizer, and the avoidance of turbulences and extra-pulmonary drug losses [33]. Supervision of the whole procedure is important for an uncomplicated and successful performance to ensure optimal and safe antibiotic nebulization in MV patients.
3 Selection of Nebulizer, Adjustment of Ventilator Settings, and Management of Circuit Parameters
One of the most important aspects of antibiotic nebulization in MV patients is the selection of the aerosol generator. Optimal drug concentration at the site of infection is clearly associated with aerosol particles of 1–5 µm in diameter (measured as mass median aerodynamic diameter or volumetric median diameter). This is the particle size that ensures maximal antibiotic delivery in the alveoli, satisfactory delivery in the distal bronchi, and minimal extra-pulmonary drug loss in the trachea and the ventilator circuit [6, 36,37,38]. The deposition of the drug is influenced also by the humidity in the ventilator circuit that may increase droplet size in the endotracheal tube and the presence of rough inner surfaces or sharp angles promoting droplet impaction in the circuit [39, 40].
3.1 Types of Generators
As a general principle, nebulizers are connected to the ventilator’s circuit and deliver a specific fraction of the drug that was inserted in their drug chamber (nominal drug dose), following aerosolization of the drug solution. Drug losses ensue in the chamber and in the ventilator’s circuit, whereas fractions of the delivered dose (the actual dose that escapes the device) is retained in the trachea and the bronchi or exhaled. Finally, only a fraction of the nominal drug dose reaches the pulmonary tissue, which represents the net capacity of the drug delivery of the device [6, 18, 41]. Currently, the available devices for inhalation of antibiotics include jet, ultrasonic, and vibrating mesh nebulizers (Table 1).
Jet nebulizers are the most commonly used because of their easiness in use, low cost, and single-use convenience without the need of disinfection between patients [6, 36,37,38,39]. They can operate with an external gas source or as ventilator-integrated systems. As external gas source may interfere with the ventilator’s gas flow causing drug turbulences, ventilator-integrated systems are preferred [6, 36]. The generated aerosol particle size is suboptimal and non-homogenous, resulting in a net capacity of drug delivery approximately 15% of the nominal drug dose [39, 40, 42].
Ultrasonic nebulizers, using a piezo-electric vibrating source, produce variable droplet sizes depending on the amplitude and frequency of the vibration. Heat generated in the drug chamber during the procedure may have unpredictable effects on its physicochemical and pharmacological properties [43]. Furthermore, ultrasonic nebulizers are characterized by a high acquisition cost and a large bedside size, large residual drug volume, inability to aerosolize viscous solutions, and the need of disinfection between patients because they are multiple-use devices [6, 36, 44]. They have a rapid delivery capacity reaching 30–40% of the nominal drug dose. As with jet nebulizers, non-homogeneous droplet size explains drug losses, with medium-sized particles (>3 µm) being deposited in the tracheal tree and proximal bronchi and larger particles (>5 µm) being impacted in the ventilator circuit or the endotracheal tube, particularly in the presence of humidification and sharp angles [39, 45].
Currently, vibrating mesh nebulizers provide the best-available nebulization performance because they produce homogenous and optimal-size drug particles, thus ensuring rapid and maximal deposition in the alveoli [6, 10, 37]. Novel vibrating mesh nebulizers are single use, not requiring disinfection between patients. However, cleaning of the device from congregating particles around the vibrating element’s apertures may prove copious. Probably, with a drug-delivery capacity in the range of 40–60% of the drug’s nominal dose, the only disadvantage of vibrating mesh nebulizers remains the high acquisition cost [6, 37, 46, 47].
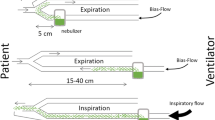
The evolution of vibrating mesh nebulizers, in the form of drug-device combinations such as the ‘pulmonary drug-delivery system’ (PDDS NKTR-061 BAYER® Inhale Program) and the ‘eFlow Rapid Nebuliser System®’ (PARI GmbH, Starnberg, Germany) is of great interest. NKTR-061 is a disposable device designed to integrate a new formulation of Amikacin Inhalation Solution (BAY41-6551 Inhale Program) into the lungs [48]. The PDDS senses the patient’s breaths and synchronizes nebulization without interfering with humidification. It is characterized by antibiotic delivery reaching 60% of the nominal drug dose and minimal lung irritation and risk of bronchospasm, and the possibility to be used after extubation as a hand-held configuration [6, 10, 44, 47, 49] (Fig. 1).
The PARI system is a single-patient multiple-use device developed to deliver a combination of fosfomycin and amikacin (five to two ratio). PARI is placed in the inspiratory arm of the circuit while a stainless steel vibrating ejector is placed at the same axis with the air flow and operates continuously [50]. Particle diameter increases from 2.8 to 3.2 mm, when exposed to circuit humidification; however, it remains adequately small for efficient delivery. Nebulization with the PARI releases efficiently approximately 90% of the charge dose; including drug solutions with high viscosity [51] (Fig. 2). PARI was used in a phase I trial including patients with VAP in whom a combination of amikacin hydrochloride with fosfomycin was administered, achieving an efficient pulmonary delivery in a short time frame of 12 min without any adverse events [52].
3.2 Connection of Nebulizer and Ventilator Settings
The principal purpose of connection and ventilator adjustments is to ensure perfect synchronization of the patient with the nebulizing device and the elimination of drug impaction in the tubing and large airways. A thorough review of the published literature has shown that the following settings have been associated with optimal drug deposition: volume-controlled mode, constant inspiratory flow, tidal volume of 8 mL/kg, and inspiratory to expiratory ratio ≤50%. In addition, the deposition of particles in the lung is facilitated by an end-inspiratory pause of 20% of the duty cycle [6, 31, 32, 42, 53]. Particular ventilator settings and connections for each type of nebulizer are presented in Table 1. An advancement with the new drug-delivery devices is the ability to maintain humidifiers during nebulization. However, the heat and moisture exchanger filter should be removed from the system during every nebulization because it affects hygroscopic features of the drug particles promoting extra-pulmonary droplet loss. It is important to mention that the expiratory bacterial/viral filter has to be changed after each nebulization irrespective of the device used. Occlusion of this filter by inevitable congregation of particles in the expiratory limb can be the cause of life-threatening complications [47].
4 Drug and Dose Selection
The selection of the inhaled antimicrobial should encompass several properties and characteristics to achieve maximum effectiveness including (1) activity against the causative pathogen; (2) physical properties to ensure maximal pulmonary delivery and minimal extra-pulmonary loss; and (3) the achievement of adequate concentrations in the lung well above the pathogen’s MIC, taking into account the need for the prevention of resistance and the presence of biofilm [10, 17].
Among multiple antimicrobials that have been used through nebulization, only colistin, tobramycin, and aztreonam possess formulations approved for aerosolized use and particularly in patients with CF [6, 10, 17]. The ideal inhaled antibiotic should be preservative free, non-hyperosmotic, and pyrogen free; possess a pH ranging from 4.0 to 8.0, an osmolarity ranging from 150 to 1200 mOsm/L, and contain at least 30 mEq of permeant anions [54]. The latter is important to prevent bronchospasm, cough, or occlusion of the expiratory filter, as in the case of ceftazidime [55]. In contrast to optimal properties for gastrointestinal absorption, moderately lipophilic and positively charged small molecules (such as tobramycin) are preferable for inhaled use [56, 57]. Viscosity should be taken into consideration as it may be incompatible with ultrasonic nebulizers and some first-generation vibrating mesh nebulizers [51]. Ideally, the volume of the diluted drug should not exceed the device’s chamber capacity, to ensure a reasonable and convenient administration time. If this is not the case, multiple consecutive nebulizations will be required [6, 10]. The evolution of continuous nebulization systems could also assist with high-volume nebulizations.
The appropriate dosage of inhaled antibiotics remains a matter of controversy, and pharmacokinetic/pharmacodynamic properties differ greatly among antibiotics. The most well-studied antibiotics are colistin and aminoglycosides. Early studies of these antibiotics intended for i.v. use were performed with the formulation intended for i.v. use administered in empirical doses via inhalation [35, 58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74]. As data from clinical trials accumulate, we will be able to gain further insight into the correct dose selection. Trials assessing various dosages of the aforementioned antibiotics are summarized in Table 2 [35, 58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74].
Colistin dosed at less than 2 million IU of colistimethate sodium using a vibrating mesh nebulizer achieved inadequate drug concentrations in the epithelial lining fluid [75]. In two recent studies, the administration of 4 million IU of inhaled colistin as monotherapy and 5 million IU every 8 days as monotherapy or in combination with intravenous aminoglycosides yielded similar results as compared to intravenous colistin and other conventional treatment, respectively, without increased nephrotoxicity [35, 58].
When administered systemically, aminoglycosides need to be administered in high doses to achieve concentrations well above the MIC owing to their reduced penetration into the interstitial space [76]. Although their efficacy in combination with other intravenous antibiotics is well known, studies assessing their administration via inhalation against MDR Gram-negative pathogens are lacking. The advent of a proprietary drug device for amikacin (PDDS) will facilitate dose selection to a standardized manner. PDDS achieved concentrations of amikacin over 6400 μg/mL in tracheal aspirates, which is equal to greater than 25 times the maximum MIC for amikacin, in half of the patients [63].
Fosfomycin is never administered as monotherapy because of the rapid emergence of resistance [77,78,79]. The relevant proprietary drug device combination (PARI eflow®) achieved high concentrations for amikacin and fosfomycin of 12,390 ± 3986 and 6174 ± 2548 μg/g, respectively [80,81,82]. Pharmacokinetic/pharmacodynamic data obtained with the novel drug-device combinations are heralding a new era in the microbiology of respiratory infections in MV patients. Based on the local antibiotic concentrations after nebulization and experience in CF, conventional in-vitro susceptibility testing may no longer predict the clinical efficacy of inhaled antibiotics, probably necessitating the implementation of “lung infection specific breakpoints” [83].
Inhaled ceftazidime and amikacin combined with intravenous antibiotics was not found to be superior to intravenous treatment in patients with VAP due to P. aeruginosa [68]. A trial assessing inhaled doripenem was interrupted in phase II as a result of pneumonitis and other allergic reactions [84]. Studies assessing inhaled vancomycin are very scarce. Two randomized placebo-controlled trials from a single center employing 120 mg of vancomycin delivered every 8 h through a vibrating mesh device, showed superior bacterial eradication and reduced acquired resistance in MV patients with ventilator-associated tracheobronchitis and VAP compared with the counterparts treated with conventional i.v. regimens [85, 86]. A considerable portion of patients were also receiving inhaled aminoglycosides based on a sputum Gram stain.
Finally, the integration of pharmacokinetic/pharmacodynamic data into daily practice may place inhaled antibiotics in the context of different therapeutic strategies. Although inhaled aminoglycosides cross inflamed lung tissues, they are not systemically accumulated [30, 87]. However, i.v. co-administration mandates the monitoring of serum levels to avoid systemic toxicity. Probably, the most suitable strategy for inhaled aminoglycosides is as adjunctive treatment to a different agent administered intravenously. However, colistin does not cross lung membranes either when administered intravenously, or when given as an inhaled form in an inflamed lung [6]. As an adjunctive treatment of i.v. administered colistin it will cause fewer considerations for incremental toxicity. However, with accumulating evidence, inhaled colistin may also represent a candidate for adjunctive treatment to another systemic antibiotic, avoiding systemic colistin administration. An absolute inhaled-only therapeutic approach (without systemically administered antibiotics) remains the ultimate challenge for inhaled antibiotics. Encouraging data have been reported only from single-center studies, although jeopardized by the well-recognized lack of a reliable definition for VAP; further large-scale randomized trials are needed [6, 35, 85, 86].
5 Patient Selection and Preparation
Optimal deposition of the drug requires perfect synchronization of the patient with the ventilator. Quite often, a patient on spontaneous breathing should step back to sedation, whereas a difficult-to-sedate patient may require to be almost paralyzed to achieve synchrony with the ventilator. This may prolong days on mechanical ventilation by delaying the weaning procedure [6, 10, 68]. Patient characteristics that may significantly impede efficient nebulization include severe bronchoconstriction, abundant viscous secretions, and spontaneous respiratory pattern or the presence of intrinsic positive end-expiratory pressure [6, 88]. Patients with asthma or acute respiratory distress syndrome (ARDS) may be less suitable for nebulization, owing to the difficulty in adjusting the necessary ventilator settings and tendency to develop complications with increased inspiratory time or tidal volume required for an efficacious nebulization [6, 37, 46, 89, 90]. In this clinical scenario, the decision to use nebulization should be made on an individual patient basis and carefully supervised [91].
Suctioning of secretions is an important step before initiating nebulization, otherwise, abundant secretions may entrap antibiotics, occlude the expiratory filter, or delay drug delivery. Interruption of the procedure for suctioning is not advisable [6, 10, 37, 46]. Severe hypoxia before nebulization may predispose to episodes of de-recruitment during nebulization, particularly in patients with a PaO2/FiO2 ratio <200 [68]. In severely hypoxic patients, prior recruitment maneuvers can be considered on an individual patient basis for exclusion from nebulization [53]. Premedication with β2-agonists to avoid bronchoconstriction is generally adopted in some studies [61, 69, 82] without being evaluated in randomized controlled trials (RCTs). This practice is advisable however in patients with a history of pre-existing bronchial hyperreactivity [asthma or chronic obstructive pulmonary disease (COPD)] [91] and in those who developed bronchoconstriction with prior antibiotic nebulization and responded effectively to bronchodilators [75].
As mentioned above, the heat-moisture exchange filter should always be removed from the tubing, as it may segregate antibiotic during nebulization, whereas heated humidification should be set to ‘off’ mode, depending on the type of nebulizer. Circuit surfaces should be as smooth as possible to avoid turbulences and all unnecessary connections should be removed. A bacterial/viral filter in the expiratory limb is advisable, but should be replaced after antibiotic nebulization [6, 10, 47, 53].
A checklist would be extremely useful for every department, ensuring that all the necessary equipment is in place before onset of the procedure. Finally, the implicated personnel should be flexible to adjust procedures according to the manufacturer of the available devices and consumables. An example of a checklist for the pre-assessment of the patient and supervision throughout the procedure is presented in Fig. 3.
6 Procedure Monitoring and Safety
Adverse reactions may arise from the nebulization procedure and local or systemic actions of the administered drug.
6.1 Procedure-Related Adverse Events
An amount of droplet impaction is anticipated in the expiratory limb, entailing a risk of occlusion of the flow meter or the expiratory filter. In one study, three patients developed this complication, two of them being censored by increased peak airway pressure, whereas the third patient developed fatal cardiac arrest [68]. In a second study, a sudden expiratory valve malfunction was reported [92]. Therefore, potential blockage of the expiratory filter should be checked for during nebulization by monitoring the peak airway pressure. Replacement of the expiratory bacterial filter is advisable after each nebulization procedure [68]. Suctioning and connecting procedures before nebulization were associated with severe de-recruitment in patients with pre-nebulization severe hypoxia; therefore, patients with a PaO2/FiO2 ratio <200 and/or ARDS, pre-existing COPD, or asthma should be carefully monitored for this complication [68, 89, 90]. Patients with asthma and COPD may also deteriorate with increased inspiratory time and patients with ARDS with the increased tidal volume that is required for effective nebulization [68, 89, 90]. Sedation-related consequences should also be taken into account, and caution is required so as to terminate nebulization without delay and return the patient rapidly to previous sedation levels if required [6, 68].
6.2 Local Antibiotic-Related Adverse Reactions
Bronchial reactivity to the inhaled antibiotic expressed as bronchospasm, cough, and chest tightness is the most common drug-related adverse event with nebulization. In patients with CF, it affected 39–45% of those receiving either tobramycin or colistin in an RCT, whereas higher rates were encountered with colistin dry powder [92,93,94,95,96,97,98]. Patients with a history of preexisting bronchial hyperreactivity (asthma or COPD) require close monitoring and premedication with inhaled β2-agonists [99]. In the most recent RCT, 2.7% of inhaled colistin-treated patients developed bronchospasm. In all cases, β2-agonists were used without recurrence [58]. Two cautionary reports of direct lung toxicity with colistin are of concern. In the first, a 5-week-old pharmacy-compounded colistin solution was responsible for the development of ARDS, attributed to the conversion of colistimethate sodium to the active and toxic form of colistin. A US Food and Drug Administration warning calls for freshly prepared solutions of colistin when using non-commercial compounds for inhalation [100, 101]. In the second, hypersensitivity pneumonia emerged after 12 days of high-dose inhaled colistin in another patient [102]. Finally, β-lactams were associated with allergic reactions from the lung and a trial of doripenem was halted because of severe bronchoconstriction [12, 84]. Clinicians should be cautious with the use of parenteral formulations of antibiotics and administer, when possible, formulations designed for inhaled use; the optimized physicochemical properties of the latter ensure better pulmonary delivery, better compatibility with aerosol generators, and less risk for toxicity.
6.3 Systemic Toxicity Due to Antibiotic Absorption
When administering high-dose inhaled aminoglycosides, serum levels should be monitored to avoid possible nephrotoxicity due to systemic accumulation, given the fact that aminoglycosides are theoretically able to cross the alveolar-capillary barrier in inflamed lungs [31, 32]. The risk is almost negligible with inhaled colistin even in the presence of infection due to its anionic molecule, which is unable to cross the alveolar-capillary barrier [68, 99]. Although detectable serum levels were reported with an inhaled dose of 5 MIU twice daily, no systemic or incremental toxicity was recorded in previous studies and in meta-analyses with inhaled colistin in MV patients [12, 14, 35, 99, 103]. A proposed checklist for assessment of the patient for the safety and supervision of the procedure is presented in Fig. 3.
7 Conclusions
Antibiotic nebulization in MV patients is a modality with increasing interest and utilization among intensivists. However, published clinical experience in this field is still limited and very heterogeneous in terms of devices, antibiotics, doses, and indications. Evidently, extrapolation of results from patients with CF and animal models to human infections and especially MV patients is not straightforward. Clearly, more clinical data with inhaled antibiotics in critically ill MV patients are required and particularly RCTs employing the evolving drug-device combinations. The accumulated evidence so far has helped us to better understand the physiology, pharmacokinetics, and practical aspects of nebulization, which are important for an efficacious and safe antibiotic delivery in critically ill MV patients. Selection of the correct nebulizer, adherence to manufacturer’s technical details, correct preparation of the procedure and the patient, continuous surveillance of the session, and early recognition of an adverse event are the most important steps for clinicians using this modality. Familiarization of personnel with the technique and type of nebulizer can ensure an optimal drug deposition in lung parenchyma and avoidance of significant drug losses in the large airways and the ventilator circuit. In addition, a checklist guarantees correct and prompt preparation of the procedure, while a strictly defined monitoring predisposes to efficacious drug delivery and safety for the patient. It is of paramount importance that intensive care unit departments employing inhaled antibiotics develop local protocols to avoid misuse with possible adverse consequences.
References
Farber JE, Ross J. The use of aerosol penicillin and streptomycin in bronchopulmonary infections. Calif Med. 1950;73:214–7.
Mearns MB, Hunt GH, Rushworth R. Bacterial flora of respiratory tract in patients with cystic fibrosis, 1950-71. Arch Dis Child. 1972;47:902–7.
Feeley TW, Du Moulin GC, Hedley-Whyte J, Bushnell LS, Gilbert JP, Feingold DS. Aerosol polymyxin and pneumonia in seriously ill patients. N Engl J Med. 1975;293:471–5.
Kuhn RJ. Formulation of aerosolized therapeutics. Chest. 2001;120:94S–8S.
Mogayzel PJ Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–9.
Rouby JJ, Bouhemad B, Monsel A, Brisson H, Arbelot C, Lu Q, et al. Aerosolized antibiotics for ventilator-associated pneumonia: lessons from experimental studies. Anesthesiology. 2012;117:1364–80.
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12.
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–111.
Waters V, Smyth A. Cystic fibrosis microbiology: advances in antimicrobial therapy. J Cyst Fibros. 2015;14:551–60.
Poulakou G, Siakallis G, Tsiodras S, Arfaras-Melainis A, Dimopoulos G. Nebulized antibiotics in mechanically ventilated patients: roadmap and challenges. Expert Rev Anti Infect Ther. 2017;15:211–29.
Sole-Lleonart C, Roberts JA, Chastre J, Poulakou G, Palmer LB, Blot S, et al. Global survey on nebulization of antimicrobial agents in mechanically ventilated patients: a call for international guidelines. Clin Microbiol Infect. 2016;22:359–64.
Kollef MH, Hamilton CW, Montgomery AB. Aerosolized antibiotics: do they add to the treatment of pneumonia? Curr Opin Infect Dis. 2013;26:538–44.
Russell CJ, Shiroishi MS, Siantz E, Wu BW, Patino CM. The use of inhaled antibiotic therapy in the treatment of ventilator-associated pneumonia and tracheobronchitis: a systematic review. BMC Pulm Med. 2016;16:40.
Zampieri FG, Nassar AP Jr, Gusmao-Flores D, Taniguchi LU, Torres A, Ranzani OT. Nebulized antibiotics for ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care. 2015;19:150.
Ehrmann S, Roche-Campo F, Sferrazza Papa GF, Isabey D, Brochard L, Apiou-Sbirlea G, et al. Aerosol therapy during mechanical ventilation: an international survey. Intensive Care Med. 2013;39:1048–56.
Sole-Lleonart C, Rouby JJ, Chastre J, Poulakou G, Palmer LB, Blot S, et al. Intratracheal administration of antimicrobial agents in mechanically ventilated adults: an international survey on delivery practices and safety. Respir Care. 2016;61:1008–14.
Palmer LB. Ventilator-associated infection: the role for inhaled antibiotics. Curr Opin Pulm Med. 2015;21:239–49.
Wenzler E, Fraidenburg DR, Scardina T, Danziger LH. Inhaled antibiotics for gram-negative respiratory infections. Clin Microbiol Rev. 2016;29:581–632.
Jamal J-A, Abdul-Aziz M-H, Lipman J, Roberts JA. Defining antibiotic dosing in lung infections. Clin Pulm Med. 2013;20:121–8.
Honeybourne D, Baldwin DR. The site concentrations of antimicrobial agents in the lung. J Antimicrob Chemother. 1992;30:249–60.
Burkhardt O, Rauch K, Kaever V, Hadem J, Kielstein JT, Welte T. Tigecycline possibly underdosed for the treatment of pneumonia: a pharmacokinetic viewpoint. Int J Antimicrob Agents. 2009;34:101–2.
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–83.
Canton R, Morosini MI. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol Rev. 2011;35:977–91.
Ni W, Wei C, Zhou C, Zhao J, Liang B, Cui J, et al. Tigecycline-Amikacin combination effectively suppresses the selection of resistance in clinical isolates of KPC-producing Klebsiella pneumoniae. Front Microbiol. 2016;7:1304.
Sinel C, Jaussaud C, Auzou M, Giard JC, Cattoir V. Mutant prevention concentrations of daptomycin for Enterococcus faecium clinical isolates. Int J Antimicrob Agents. 2016;48:449–52.
Gil-Perotin S, Ramirez P, Marti V, Sahuquillo JM, Gonzalez E, Calleja I, et al. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: a state of concept. Crit Care. 2012;16:R93.
Bercault N, Boulain T. Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: a prospective case-control study. Crit Care Med. 2001;29:2303–9.
Luna CM, Aruj P, Niederman MS, Garzon J, Violi D, Prignoni A, et al. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J. 2006;27:158–64.
Elman M, Goldstein I, Marquette CH, Wallet F, Lenaour G, Rouby JJ, et al. Influence of lung aeration on pulmonary concentrations of nebulized and intravenous amikacin in ventilated piglets with severe bronchopneumonia. Anesthesiology. 2002;97:199–206.
Ferrari F, Goldstein I, Nieszkowszka A, Elman M, Marquette CH, Rouby JJ, et al. Lack of lung tissue and systemic accumulation after consecutive daily aerosols of amikacin in ventilated piglets with healthy lungs. Anesthesiology. 2003;98:1016–9.
Goldstein I, Wallet F, Nicolas-Robin A, Ferrari F, Marquette CH, Rouby JJ. Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med. 2002;166:1375–81.
Goldstein I, Wallet F, Robert J, Becquemin MH, Marquette CH, Rouby JJ. Lung tissue concentrations of nebulized amikacin during mechanical ventilation in piglets with healthy lungs. Am J Respir Crit Care Med. 2002;165:171–5.
Luyt CE, Brechot N, Combes A, Trouillet JL, Chastre J. Delivering antibiotics to the lungs of patients with ventilator-associated pneumonia: an update. Expert Rev Anti Infect Ther. 2013;11:511–21.
Tonnellier M, Ferrari F, Goldstein I, Sartorius A, Marquette CH, Rouby JJ. Intravenous versus nebulized ceftazidime in ventilated piglets with and without experimental bronchopneumonia: comparative effects of helium and nitrogen. Anesthesiology. 2005;102:995–1000.
Lu Q, Luo R, Bodin L, Yang J, Zahr N, Aubry A, et al. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology. 2012;117:1335–47.
Ari A, Areabi H, Fink JB. Evaluation of aerosol generator devices at 3 locations in humidified and non-humidified circuits during adult mechanical ventilation. Respir Care. 2010;55:837–44.
Bassetti M, Luyt CE, Nicolau DP, Pugin J. Characteristics of an ideal nebulized antibiotic for the treatment of pneumonia in the intubated patient. Ann Intensive Care. 2016;6:35.
Ehrmann S, Lyazidi A, Louis B, Isabey D, Le Pennec D, Brochard L, et al. Ventilator-integrated jet nebulization systems: tidal volume control and efficiency of synchronization. Respir Care. 2014;59:1508–16.
Miller DD, Amin MM, Palmer LB, Shah AR, Smaldone GC. Aerosol delivery and modern mechanical ventilation: in vitro/in vivo evaluation. Am J Respir Crit Care Med. 2003;168:1205–9.
Pritchard JN. The influence of lung deposition on clinical response. J Aerosol Med. 2001;14:S19–26.
Patton JS, Brain JD, Davies LA, Fiegel J, Gumbleton M, Kim KJ, et al. The particle has landed–characterizing the fate of inhaled pharmaceuticals. J Aerosol Med Pulm Drug Deliv. 2010;23:S71–87.
Ferrari F, Lu Q, Girardi C, Petitjean O, Marquette CH, Wallet F, et al. Nebulized ceftazidime in experimental pneumonia caused by partially resistant Pseudomonas aeruginosa. Intensive Care Med. 2009;35:1792–800.
Takanami C, Goto Y. Physical properties of antibiotic aerosols produced by jet and ultrasonic nebulizers. J Aerosol Med. 1990;3:45–52.
Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, Fink JB. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010;55:845–51.
Laube BL, Janssens HM, de Jongh FH, Devadason SG, Dhand R, Diot P, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–31.
Dhand R. Aerosol delivery during mechanical ventilation: from basic techniques to new devices. J Aerosol Med Pulm Drug Deliv. 2008;21:45–60.
Luyt CE, Clavel M, Guntupalli K, Johannigman J, Kennedy JI, Wood C, et al. Pharmacokinetics and lung delivery of PDDS-aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia. Crit Care. 2009;13:R200.
Clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT01969799. Accessed 2 Aug 2016.
Kadrichu N BS, Corkery K, et al. In vitro efficiency of the Amikacin Inhale System, a novel integrated drug-device delivery system. ISICEM, 19–22 March 2013, Poster A384.
PARI eFlow rapid, Technical Data. Available from: http://wwwparide/uk-en/products/lowerairways-1/eflow-rapid-nebuliser-system-1/. Accessed 2 Aug 2016.
Ari A. Jet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol. 2014;16:1–7.
Montgomery AB, Vallance S, Abuan T, Tservistas M, Davies A. A randomized double-blind placebo-controlled dose-escalation phase 1 study of aerosolized amikacin and fosfomycin delivered via the PARI investigational eFlow(R) inline nebulizer system in mechanically ventilated patients. J Aerosol Med Pulm Drug Deliv. 2014;27:441–8.
Rello J, Rouby JJ, Sole-Lleonart C, Chastre J, Blot S, Luyt CE, et al. Key conceptional considerations on nebulization of antimicrobial agents to mechanically ventilated patients: a consensus statement from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect. 2017;S1198–743X:30187–8.
Le J, Ashley ED, Neuhauser MM, Brown J, Gentry C, Klepser ME, et al. Consensus summary of aerosolized antimicrobial agents: application of guideline criteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2010;30:562–84.
Eschenbacher WL, Boushey HA, Sheppard D. Alteration in osmolarity of inhaled aerosols cause bronchoconstriction and cough, but absence of a permeant anion causes cough alone. Am Rev Respir Dis. 1984;129:211–5.
Byron PR. Physicochemical effects on lung disposition of pharmaceutical aerosols. Aerosol Sci Tech. 1993;18:223–9.
Montgomery ABPW, Nardella P, et al. Sputum concentrations and systemic pharmacokinetics of aerosolized tobramycin (Tobi) in diseased lungs. Respir Drug Deliv. 2000;1:19–24.
Abdellatif S, Trifi A, Daly F, Mahjoub K, Nasri R, Ben Lakhal S. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care. 2016;6:26.
Arnold HM, Sawyer AM, Kollef MH. Use of adjunctive aerosolized antimicrobial therapy in the treatment of Pseudomonas aeruginosa and Acinetobacter baumannii ventilator-associated pneumonia. Respir Care. 2012;57:1226–33.
Falagas ME, Siempos II, Rafailidis PI, Korbila IP, Ioannidou E, Michalopoulos A. Inhaled colistin as monotherapy for multidrug-resistant gram (−) nosocomial pneumonia: a case series. Respir Med. 2009;103:707–13.
Ghannam DE, Rodriguez GH, Raad II, Safdar A. Inhaled aminoglycosides in cancer patients with ventilator-associated Gram-negative bacterial pneumonia: safety and feasibility in the era of escalating drug resistance. Eur J Clin Microbiol Infect Dis. 2009;28:253–9.
Kalin G, Alp E, Coskun R, Demiraslan H, Gundogan K, Doganay M. Use of high-dose IV and aerosolized colistin for the treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia: do we really need this treatment? J Infect Chemother. 2012;18:872–7.
Kofteridis DP, Alexopoulou C, Valachis A, Maraki S, Dimopoulou D, Georgopoulos D, et al. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: a matched case-control study. Clin Infect Dis. 2010;51:1238–44.
Kollef MH, Ricard JD, Roux D, Francois B, Ischaki E, Rozgonyi Z, et al. A randomized trial of the amikacin fosfomycin inhalation system for the adjunctive therapy of gram-negative ventilator-associated pneumonia: IASIS trial. Chest. 2017;151:1239–46.
Korbila IP, Michalopoulos A, Rafailidis PI, Nikita D, Samonis G, Falagas ME. Inhaled colistin as adjunctive therapy to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin Microbiol Infect. 2010;16:1230–6.
Kuo SC, Lee YT, Yang SP, Chen CP, Chen TL, Hsieh SL, et al. Eradication of multidrug-resistant Acinetobacter baumannii from the respiratory tract with inhaled colistin methanesulfonate: a matched case-control study. Clin Microbiol Infect. 2012;18:870–6.
Lin CC, Liu TC, Kuo CF, Liu CP, Lee CM. Aerosolized colistin for the treatment of multidrug-resistant Acinetobacter baumannii pneumonia: experience in a tertiary care hospital in northern Taiwan. J Microbiol Immunol Infect. 2010;43:323–31.
Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ, et al. Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2011;184:106–15.
Michalopoulos A, Fotakis D, Virtzili S, Vletsas C, Raftopoulou S, Mastora Z, et al. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: a prospective study. Respir Med. 2008;102:407–12.
Michalopoulos A, Kasiakou SK, Mastora Z, Rellos K, Kapaskelis AM, Falagas ME. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit Care. 2005;9:R53–9.
Niederman MS, Chastre J, Corkery K, Fink JB, Luyt CE, Garcia MS. BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with Gram-negative pneumonia. Intensive Care Med. 2012;38:263–71.
Pereira GH, Muller PR, Levin AS. Salvage treatment of pneumonia and initial treatment of tracheobronchitis caused by multidrug-resistant Gram-negative bacilli with inhaled polymyxin B. Diagn Microbiol Infect Dis. 2007;58:235–40.
Rattanaumpawan P, Lorsutthitham J, Ungprasert P, Angkasekwinai N, Thamlikitkul V. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother. 2010;65:2645–9.
Zah Bogovic TBR, Tomasevic B, et al. Inhalation plus intravenous colistin versus intravenous colistin alone for treatment of ventilator associated pneumonia. Signa Vitae. 2014;9:29–33.
Boisson M, Jacobs M, Gregoire N, Gobin P, Marchand S, Couet W, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother. 2014;58:7331–9.
Rodvold KA, George JM, Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet. 2011;50:637–64.
Rodriguez-Rojas A, Macia MD, Couce A, Gomez C, Castaneda-Garcia A, Oliver A, et al. Assessing the emergence of resistance: the absence of biological cost in vivo may compromise fosfomycin treatments for P. aeruginosa infections. PLoS One. 2010;5:e10193.
Souli M, Galani I, Boukovalas S, Gourgoulis MG, Chryssouli Z, Kanellakopoulou K, et al. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob Agents Chemother. 2011;55:2395–7.
Walsh CC, Landersdorfer CB, McIntosh MP, Peleg AY, Hirsch EB, Kirkpatrick CM, et al. Clinically relevant concentrations of fosfomycin combined with polymyxin B, tobramycin or ciprofloxacin enhance bacterial killing of Pseudomonas aeruginosa, but do not suppress the emergence of fosfomycin resistance. J Antimicrob Chemother. 2016;71:2218–29.
Montgomery AB, Rhomberg PR, Abuan T, Walters KA, Flamm RK. Amikacin-fosfomycin at a five-to-two ratio: characterization of mutation rates in microbial strains causing ventilator-associated pneumonia and interactions with commonly used antibiotics. Antimicrob Agents Chemother. 2014;58:3708–13.
Montgomery AB, Rhomberg PR, Abuan T, Walters KA, Flamm RK. Potentiation effects of amikacin and fosfomycin against selected amikacin-nonsusceptible Gram-negative respiratory tract pathogens. Antimicrob Agents Chemother. 2014;58:3714–9.
Trapnell BC, McColley SA, Kissner DG, Rolfe MW, Rosen JM, McKevitt M, et al. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. Am J Respir Crit Care Med. 2012;185:171–8.
Hurley MN, Ariff AH, Bertenshaw C, Bhatt J, Smyth AR. Results of antibiotic susceptibility testing do not influence clinical outcome in children with cystic fibrosis. J Cyst Fibros. 2012;11:288–92.
Agency TEM. Summary of product characteristics of Doripenem, at Doribax : EPAR—Product Information—EMA—Europa.eu. https://www.google.gr/url?sa=t%26rct=j%26q=%26esrc=s%26source=web%26cd=4%26ved=0ahUKEwjmr7nTw-bTAhXjHJoKHWooD2QQFgg9MAM%26url=http%3A%2F%2Fwww.ema.europa.eu%2Fdocs%2Fen_GB%2Fdocument_library%2FEPAR_-_Product_Information%2Fhuman%2F000891%2FWC500037148.pdf%26usg=AFQjCNEWnxojzDOSm-Lp_rdlfHH5fFRu8g%26sig2=vA2OwtLVK7swLcPBcPMjwA%26cad=rja. Accessed 2 Aug 2016.
Palmer LB, Smaldone GC. Reduction of bacterial resistance with inhaled antibiotics in the intensive care unit. Am J Respir Crit Care Med. 2014;189:1225–33.
Palmer LB, Smaldone GC, Chen JJ, Baram D, Duan T, Monteforte M, et al. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit Care Med. 2008;36:2008–13.
Le Conte P, Potel G, Peltier P, Horeau D, Caillon J, Juvin ME, et al. Lung distribution and pharmacokinetics of aerosolized tobramycin. Am Rev Respir Dis. 1993;147:1279–82.
Laghi F, Goyal A. Auto-PEEP in respiratory failure. Min Anestesiol. 2012;78:201–21.
Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1.
Parrilla FJ, Moran I, Roche-Campo F, Mancebo J. Ventilatory strategies in obstructive lung disease. Semin Respir Crit Care Med. 2014;35:431–40.
Soltaninejad F, Kheiri S, Habibian R, Amra A, Asgari-Savadjani S. Evaluation effects of nebulized gentamicin in exacerbation of chronic obstructive lung disease. J Res Med Sci. 2016;21:56.
Mojoli F, Iotti GA, Imberti R, Braschi A. The importance of protecting the mechanical ventilator during colistin methanesulfonate nebulization. Intensive Care Med. 2013;39:535–6.
Cunningham S, Prasad A, Collyer L, Carr S, Lynn IB, Wallis C. Bronchoconstriction following nebulised colistin in cystic fibrosis. Arch Dis Child. 2001;84:432–3.
Dodd ME, Abbott J, Maddison J, Moorcroft AJ, Webb AK. Effect of tonicity of nebulised colistin on chest tightness and pulmonary function in adults with cystic fibrosis. Thorax. 1997;52:656–8.
Hodson ME, Gallagher CG, Govan JR. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J. 2002;20:658–64.
Konstan MW, Flume PA, Kappler M, Chiron R, Higgins M, Brockhaus F, et al. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: the EAGER trial. J Cyst Fibros. 2011;10:54–61.
Maddison J, Dodd M, Webb AK. Nebulized colistin causes chest tightness in adults with cystic fibrosis. Respir Med. 1994;88:145–7.
Schuster A, Haliburn C, Doring G, Goldman MH, Freedom Study G. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax. 2013;68:344–50.
Dominguez-Ortega J, Manteiga E, Abad-Schilling C, Juretzcke MA, Sanchez-Rubio J, Kindelan C. Induced tolerance to nebulized colistin after severe reaction to the drug. J Investig Allergol Clin Immunol. 2007;17:59–61.
Food And Drug Administration. Information for Healthcare Professionals: Colistimethate; an alert. Posted in 2007. Accessed 30 Aug 2016.
McCoy KS. Compounded colistimethate as possible cause of fatal acute respiratory distress syndrome. N Engl J Med. 2007;357:2310–1.
Leong KW, Ong S, Chee HL, Lee W, Kwa AL. Hypersensitivity pneumonitis due to high-dose colistin aerosol therapy. Int J Infect Dis. 2010;14:e1018–9.
Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, et al. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother. 2006;57:306–11.
Acknowledgements
We would like to thank Dr I.T. Virlos for the linguistic revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
GP, DKM, DPN, GS, and GD have no conflicts of interest directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Poulakou, G., Matthaiou, D.K., Nicolau, D.P. et al. Inhaled Antimicrobials for Ventilator-Associated Pneumonia: Practical Aspects. Drugs 77, 1399–1412 (2017). https://doi.org/10.1007/s40265-017-0787-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0787-0