Abstract
Eslicarbazepine acetate (Aptiom®) is a once-daily, orally administered antiepileptic drug (AED) approved previously in the EU, USA and several other countries for use as adjunctive therapy for the treatment of partial-onset seizures. Based on the findings of two randomized, dose-blinded, conversion-to-monotherapy phase III trials in patients with uncontrolled partial epilepsy, the US license for eslicarbazepine acetate has recently been expanded to include use as monotherapy for partial-onset seizures. The pivotal trials demonstrated that seizure control following conversion from other AEDs was superior for eslicarbazepine acetate monotherapy (1200 or 1600 mg once daily) compared with a pseudo-placebo historical control. Other efficacy outcomes appeared to support the benefit of treatment, with up to 10 % of patients remaining seizure free and up to 46 % of patients experiencing a ≥50 % reduction from baseline in standardized seizure frequency during the monotherapy periods of the trials. Eslicarbazepine acetate monotherapy was generally well tolerated, with most treatment-emergent adverse events being mild to moderate in severity. Its tolerability profile was generally consistent with the established profile of the drug based on its use as adjunctive therapy. Thus, once-daily eslicarbazepine acetate, either as monotherapy or adjunctive therapy, represents a useful option for the treatment of patients with partial-onset seizures. The recent licensing of the drug in the USA as monotherapy expands the range of treatment options for patients with partial-onset seizures and increases the opportunity to tailor therapy to the individual patient.

Similar content being viewed by others
References
Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
World Health Organization. Epilepsy—fact sheet no. 999. 2015. http://www.who.int/mediacentre/factsheets/fs999/en/#. Accessed 14 Mar 2016.
Centers for Disease Control and Prevention. Epilepsy in adults and access to care—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(45):909–13.
Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–85.
Goldenberg MM. Overview of drugs used for epilepsy and seizures: etiology, diagnosis, and treatment. P T. 2010;35(7):392–415.
Duncan JS, Sander JW, Sisodiya SM, et al. Adult epilepsy. Lancet. 2006;367(9516):1087–100.
Maguire M, Marson A, Ramaratnam S. Epilepsy (partial). BMJ Clin Evid. 2011;5(1214):1–43.
St Louis EK. Minimizing AED adverse effects: improving quality of life in the interictal state in epilepsy care. Curr Neuropharmacol. 2009;7(2):106–14.
Moshé SL, Perucca E, Ryvlin P, et al. Epilepsy: new advances. Lancet. 2015;385(9971):884–98.
French JA, Schachter S. A workshop on antiepileptic drug monotherapy indications. Epilepsia. 2002;43(Suppl 10):3–27.
St Louis EK, Rosenfeld WE, Bramley T. Antiepileptic drug monotherapy: the initial approach in epilepsy management. Curr Neuropharmacol. 2009;7(2):77–82.
US FDA. Aptiom® (eslicarbazepine acetate) tablets: US prescribing information. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022416s001lbl.pdf. Accessed 14 Mar 2016.
Benes J, Parada A, Figueiredo AA, et al. Anticonvulsant and sodium channel-blocking properties of novel 10,11-dihydro-5H-dibenz[b, f]azepine-5-carboxamide derivatives. J Med Chem. 1999;42(14):2582–7.
Almeida L, Soares-da-Silva P. Eslicarbazepine acetate (BIA 2-093). Neurotherapeutics. 2007;4(1):88–96.
Nunes T, Rocha JF, Falcão A, et al. Steady-state plasma and cerebrospinal fluid pharmacokinetics and tolerability of eslicarbazepine acetate and oxcarbazepine in healthy volunteers. Epilepsia. 2013;54(1):108–16.
Bialer M, Soares-da-Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia. 2012;53(6):935–46.
Eurpoean Medicines Agency. Zebinix: summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000988/WC500047225.pdf. Accessed 14 Mar 2016.
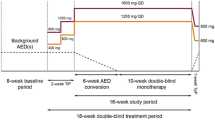
Jacobson MP, Pazdera L, Bhatia P, et al. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a historical-control phase III study. BMC Neurol. 2015;15:46.
Sperling MR, Harvey J, Grinnell T, et al. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America. Epilepsia. 2015;56(4):546–55.
Keating GM. Eslicarbazepine acetate: a review of its use as adjunctive therapy in refractory partial-onset seizures. CNS Drugs. 2014;28(7):583–600.
Soares-da-Silva P, Pires N, Bonifácio MJ, et al. Eslicarbazepine acetate for the treatment of focal epilepsy: an update on its proposed mechanisms of action. Pharmacol Res Perspect. 2015;3(2):e00124.
Bonifácio MJ, Sheridan RD, Parada A, et al. Interaction of the novel anticonvulsant, BIA 2-093, with voltage-gated sodium channels: comparison with carbamazepine. Epilepsia. 2001;42(5):600–8.
Hebeisen S, Brady K, Konrad D, et al. Inhibitory effects of eslicarbazepine acetate and its metabolites against neuronal voltage-gated sodium channels [abstract no. p851]. Epilepsia. 2011;52(Suppl 6):257–8.
Brady K, Hebeisen S, Konrad D, et al. The effects of eslicarbazepine, R-licarbazepine, oxcarbazepine and carbamazepine on ion transmission through Cav3.2 channels [abstract no. p858]. Epilepsia. 2011;52(Suppl 6):260.
Pires N, Palma N, Loureiro AI, et al. Effects of eslicarbazepine acetate, eslicarbazepine, carbamazepine and oxcarbazepine in the maximal electroconvulsive shock test in the mice [abstract no. p373]. Epilepsia. 2011;52(Suppl 6):118.
Torrão L, Machado R, Pires N, et al. Effects of eslicarbazepine acetate, eslicarbazepine, carbamazepine and oxcarbazepine in the 6-Hz psychomotor seizure model in the mice [abstract no. p374]. Epilepsia. 2011;52(Suppl 6):118–9.
Potschka H, Soerensen J, Pekcec A, et al. Effect of eslicarbazepine acetate in the corneal kindling progression and the amygdala kindling model of temporal lobe epilepsy. Epilepsy Res. 2014;108(2):212–22.
Vaz-Da-Silva M, Nunes T, Almeida L, et al. Evaluation of eslicarbazepine acetate on cardiac repolarization in a thorough QT/QTc study. J Clin Pharmacol. 2012;52(2):222–33.
Perucca E, Elger C, Halász P, et al. Pharmacokinetics of eslicarbazepine acetate at steady-state in adults with partial-onset seizures. Epilepsy Res. 2011;96(1–2):132–9.
Maia J, Vaz-da-Silva M, Almeida L, et al. Effect of food on the pharmacokinetic profile of eslicarbazepine acetate (BIA 2-093). Drugs R D. 2005;6(4):201–6.
Almeida L, Potgieter JH, Maia J, et al. Pharmacokinetics of eslicarbazepine acetate in patients with moderate hepatic impairment. Eur J Clin Pharmacol. 2008;64(3):267–73.
Almeida L, Soares-da-Silva P. Safety, tolerability, and pharmacokinetic profile of BIA 2-093, a novel putative antiepileptic, in a rising multiple-dose study in young healthy humans. J Clin Pharmacol. 2004;44(8):906–18.
Maia J, Almeida L, Falcão A, et al. Effect of renal impairment on the pharmacokinetics of eslicarbazepine acetate. Int J Clin Pharmacol Ther. 2008;46(3):119–30.
Falcão A, Pinto R, Nunes T, et al. Effect of repeated administration of eslicarbazepine acetate on the pharmacokinetics of simvastatin in healthy subjects. Epilepsy Res. 2013;106(1–2):244–9.
Falcão A, Vaz-da-Silva M, Gama H, et al. Effect of eslicarbazepine acetate on the pharmacokinetics of a combined ethinylestradiol/levonorgestrel oral contraceptive in healthy women. Epilepsy Res. 2013;105(3):368–76.
Vaz-da-Silva M, Almeida L, Falcão A, et al. Effect of eslicarbazepine acetate on the steady-state pharmacokinetics and pharmacodynamics of warfarin in healthy subjects during a three-stage, open-label, multiple-dose, single-period study. Clin Ther. 2010;32(1):179–92.
French JA, Wang S, Warnock B, et al. Historical control monotherapy design in the treatment of epilepsy. Epilepsia. 2010;51(10):1936–43.
Perucca E. When clinical trials make history: demonstrating efficacy of new antiepileptic drugs as monotherapy. Epilepsia. 2010;51(10):1933–5.
Pazdera L, French J, Sperling MR, et al. Eslicarbazepine acetate monotherapy in adults with partial-onset seizures: a pooled analysis of two randomized, double-blind studies with use of a historical control [abstract no. 1.318 plus poster]. Epilepsy Curr. 2015;15(s1):148–9.
Krauss G, Biton V, Cheng H, et al. Improvement in seizure control during conversion to eslicarbazepine acetate monotherapy: a pooled analysis of two trials in adults with refractory partial-onset seizures [abstract no. 2.292 plus poster]. Epilepsy Curr. 2015;15(s1):324–5.
Sperling MR, French J, Jacobson MP, et al. Conversion to eslicarbazepine acetate monotherapy: a pooled analysis of 2 phase III studies. Neurology. 2016. doi:10.1212/WNL.0000000000002497.
Sperling MR, Rogin JB, Harvey JH, et al. Long-term safety and efficacy of eslicarbazepine acetate monotherapy in adults with refractory partial-onset seizures: an open-label extension study [abstract no. 2.290 plus poster]. Epilepsy Curr. 2015;15(s1):323.
Borghs S, de la Loge C, Cramer JA. Defining minimally important change in QOLIE-31 scores: estimates from three placebo-controlled lacosamide trials in patients with partial-onset seizures. Epilepsy Behav. 2012;23(3):230–4.
Gilliam F, Cheng H, Blum D. Changes in quality of life (QoL) and depressive symptoms in a long-term open-label extension (OLE) of eslicarbazepine acetate (ESL) monotherapy studies in adults with refractory partial-onset seizures (POS) [abstract no. P1.234]. Neurology. 2015;84(14 Suppl):P1.234.
French JA, Temkin NR, Shneker BF, et al. Lamotrigine XR conversion to monotherapy: first study using a historical control group. Neurotherapeutics. 2012;9(1):176–84.
Stephen LJ, Brodie MJ. Pharmacotherapy of epilepsy: newly approved and developmental agents. CNS Drugs. 2011;25(2):89–107.
Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–63.
Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 2011;10(5):446–56.
French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62(8):1261–73.
French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs I: treatment of new onset epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62(8):1252–60.
Acknowledgments
During the peer review process, the manufacturer of eslicarbazepine acetate was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
Matt Shirley and Sohita Dhillon are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: M. L. Barker - Haliski, Department of Pharmacy, University of Washington, Seattle, WA, USA; W. E. Rosenfeld, The Comprehensive Epilepsy Care Center for Children and Adults, St. Louis, MO, USA; L. Sander, Department of Clinical and Experimental Epilepsy, University College London Institute of Neurology, London, UK.
Rights and permissions
About this article
Cite this article
Shirley, M., Dhillon, S. Eslicarbazepine Acetate Monotherapy: A Review in Partial-Onset Seizures. Drugs 76, 707–717 (2016). https://doi.org/10.1007/s40265-016-0570-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0570-7