Abstract
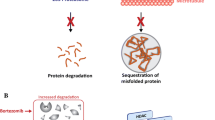
Novartis has developed oral and intravenous formulations of panobinostat (Farydak®), a histone deacetylase (HDAC) inhibitor, for the treatment of cancer. HDACs have important roles in maintaining chromatin structure and in regulating gene expression, including that of tumour suppressor genes, and thus represent valid targets in the search for cancer therapeutics. Oral panobinostat is approved in the US, as combination therapy with bortezomib and dexamethasone in patients with recurrent multiple myeloma who have received at least two prior treatment regimens, including bortezomib and an immunomodulatory agent. Regulatory submissions have been made for the use of combination therapy with panobinostat in patients with recurrent multiple myeloma in the EU and Japan. Panobinostat is in various stages of clinical development worldwide for a range of haematological and solid tumours. This article summarizes the milestones in the development of panobinostat leading to this first approval for multiple myeloma.
Similar content being viewed by others
References
Terpos E. The synergistic effect of panobinostat (LBH589) with melphalan or doxorubicin on multiple myeloma cells; rationale for the use of combination regimens in myeloma patients. Leuk Res. 2011;35(3):295–6.
Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280(2):233–41.
West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124(1):30–9.
Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol. 2007;1(1):19–25.
Mithraprabhu S, Kalff A, Chow A, et al. Dysregulated Class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics. 2014;9(11):1511–20.
Novartis Pharmaceuticals Corporation. Farydak® (panobinostat capsules): US prescribing information. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205353s000lbl.pdf. Accessed 25 Feb 2015.
US Food and Drug Administration. FDA approves Farydak for treatment of multiple myeloma [media release]. 23 Feb 2015. http://www.fda.gov/.
Novartis. Novartis receives FDA approval of Farydak®, the first HDAC inhibitor for patients with multiple myeloma [media release]. 23 Feb 2015. http://www.novartis.com/.
Novartis. Novartis announces extension to FDA review period for multiple myeloma investigational compound LBH589 [media release]. 25 Nov 2014. http://www.novartis.com.
Novartis. Novartis announces outcome of FDA advisory committee meeting for multiple myeloma investigational compound LBH589 [media release]. 6 Nov 2014. http://www.novartis.com.
Novartis. Novartis Pharmaceuticals fact sheet. 2015. http://www.novartis.com/downloads/newsroom/corporate-fact-sheet/2a_Pharmaceuticals_EN.pdf. Accessed 11 Mar 2015.
European Medicines Agency. Public summary of opinion on orphan designation: panobinostat for the treatment of multiple myeloma. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2012/12/WC500136185.pdf. Accessed 11 Mar 2015.
Floris G, Debiec-Rychter M, Sciot R, et al. High efficacy of panobinostat towards human gastrointestinal stromal tumors in a xenograft mouse model. Clin Cancer Res. 2009;15(12):4066–76.
Ocio EM, Vilanova D, Atadja P, et al. In vitro and in vivo rationale for the triple combination of panobinostat (LBH589) and dexamethasone with either bortezomib or lenalidomide in multiple myeloma. Haematologica. 2010;95(5):794–803.
Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther. 2011;10(11):2034–42.
Catley L, Weisberg E, Kiziltepe T, et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108(10):3441–9.
Giles F, Fischer T, Cortes J, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12(15):4628–35.
Govindaraj C, Tan P, Walker P, et al. Reducing TNF receptor 2+ regulatory T cells via the combined action of azacitidine and the HDAC inhibitor, panobinostat for clinical benefit in acute myeloid leukemia patients. Clin Cancer Res. 2014;20(3):724–35.
Shapiro GI, Frank R, Dandamudi UB, et al. The effect of food on the bioavailability of panobinostat, an orally active pan-histone deacetylase inhibitor, in patients with advanced cancer. Cancer Chemother Pharmacol. 2012;69(2):555–62.
Clive S, Woo MM, Nydam T, et al. Characterizing the disposition, metabolism, and excretion of an orally active pan-deacetylase inhibitor, panobinostat, via trace radiolabeled 14C material in advanced cancer patients. Cancer Chemother Pharmacol. 2012;70(4):513–22.
Slingerland M, Hess D, Clive S, et al. A phase I, open-label, multicenter study to evaluate the pharmacokinetics and safety of oral panobinostat in patients with advanced solid tumors and various degrees of hepatic function. Cancer Chemother Pharmacol. 2014;74(5):1089–98.
Porro M, Sharma S, Witteveen PO, et al. Oral panobinostat in patients with advanced tumors and impaired renal function: Relationship between pharmacokinetics and key safety parameters [abstract no. 572]. In: 26th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics. 2014.
Perez L, Fernandez HF, Tomblyn M, et al. A phase I/II trial evaluating the use of a histone deacetylase inhibitor panobinostat (LBH589) in addition to glucocorticoids in patients with acute graft-versus-host disease [abstract no. 3308]. In: 55th Annual Meeting and Exposition of the American Society of Hematology. 2013.
Bug G, Burchert A, Nicolaus K, et al. Post-transplant maintenance with the deacetylase inhibitor panobinostat in patients with high-risk AML or MDS: results of the phase I part of the Panobest trial [abstract no. 3315]. In: 55th Annual Meeting and Exposition of the American Society of Hematology. 2013.
Ribrag V, Harrison CN, Heidel FH, et al. A phase 1b, dose-finding study of ruxolitinib plus panobinostat in patients with primary myelofibrosis (PMF), post-polycythemia vera MF (PPV-MF), or post-essential thrombocythemia MF (PET-MF): identification of the recommended phase 2 dose [abstract no. 4045]. In: 55th Annual Meeting and Exposition of the American Society of Hematology. 2013.
Shi W. Phase I study of panobinostat and fractionated stereotactic re-irradiation therapy (FSRT) for recurrent high grade gliomas [abstract no. 216]. In: 26th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics. 2014.
Heidel F, Ribrag V, Vannucchi AM, et al. A phase 1b, dose-finding study of ruxolitinib plus panobinostat in patients with myelofibrosis [abstract no. 7022]. In: 50th Annual Meeting of the American Society of Clinical Oncology. 2014.
Bauer S, Hilger RA, Muhlenberg T, et al. Phase I study of panobinostat and imatinib in patients with treatment-refractory metastatic gastrointestinal stromal tumors. Br J Cancer. 2014;110(5):1155–62.
Gray JE, Haura E, Chiappori A, et al. A phase I, pharmacokinetic, and pharmacodynamic study of panobinostat, an HDAC inhibitor, combined with erlotinib in patients with advanced aerodigestive tract tumors. Clin Cancer Res. 2014;20(6):1644–55.
Berenson JR, Hilger JD, Yellin O, et al. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Ann Hematol. 2014;93(1):89–98.
Tarhini AA, Zahoor H, McLaughlin B, et al. Phase I trial of carboplatin and etoposide in combination with panobinostat in patients with lung cancer. Anticancer Res. 2013;33(10):4475–81.
San-Miguel JF, Richardson PG, Gunther A, et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol. 2013;31(29):3696–703.
DeAngelo DJ, Spencer A, Bhalla KN, et al. Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia. 2013;27(8):1628–36.
Mascarenhas J, Lu M, Li T, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF). Br J Haematol. 2013;161(1):68–75.
Jones SF, Infante JR, Thompson DS, et al. A phase I trial of oral administration of panobinostat in combination with paclitaxel and carboplatin in patients with solid tumors. Cancer Chemother Pharmacol. 2012;70(3):471–5.
Strickler JH, Starodub AN, Jia J, et al. Phase I study of bevacizumab, everolimus, and panobinostat (LBH-589) in advanced solid tumors. Cancer Chemother Pharmacol. 2012;70(2):251–8.
Drappatz J, Lee EQ, Hammond S, et al. Phase I study of panobinostat in combination with bevacizumab for recurrent high-grade glioma. J Neurooncol. 2012;107(1):133–8.
Fukutomi A, Hatake K, Matsui K, et al. A phase I study of oral panobinostat (LBH589) in Japanese patients with advanced solid tumors. Invest New Drugs. 2012;30(3):1096–106.
Jones SF, Bendell JC, Infante JR, et al. A phase I study of panobinostat in combination with gemcitabine in the treatment of solid tumors. Clin Adv Hematol Oncol. 2011;9(3):225–30.
Rathkopf D, Wong BY, Ross RW, et al. A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2010;66(1):181–9.
Berdeja JG, Hart LL, Mace JR, et al. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica. 2015;. doi:10.3324/haematol.2014.119735.
Sharma S, Beck J, Mita M, et al. A phase I dose-escalation study of intravenous panobinostat in patients with lymphoma and solid tumors. Invest New Drugs. 2013;31(4):974–85.
Morita S, Oizumi S, Minami H, et al. Phase I dose-escalating study of panobinostat (LBH589) administered intravenously to Japanese patients with advanced solid tumors. Invest New Drugs. 2012;30(5):1950–7.
San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–206.
Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122(14):2331–7.
Offidani M, Polloni C, Cavallo F, et al. Phase II study of melphalan, thalidomide and prednisone combined with oral panobinostat in patients with relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012;53(9):1722–7.
Ghobrial IM, Campigotto F, Murphy TJ, et al. Results of a phase 2 trial of the single-agent histone deacetylase inhibitor panobinostat in patients with relapsed/refractory Waldenstrom macroglobulinemia. Blood. 2013;121(8):1296–303.
DeAngelo DJ, Mesa RA, Fiskus W, et al. Phase II trial of panobinostat, an oral pan-deacetylase inhibitor in patients with primary myelofibrosis, post-essential thrombocythaemia, and post-polycythaemia vera myelofibrosis. Br J Haematol. 2013;162(3):326–35.
Zaja F, Salvi F, Franceschetti S, et al. Inhibition of histone deacetylases with panobinostat as a treatment for relapsed or refractory diffuse large B-cell lymphoma: a phase II study by the Fondazione Italiana Linfomi [abstract no. 3047]. In: 55th Annual Meeting and Exposition of the American Society of Hematology. 2013.
de Marinis F, Atmaca A, Tiseo M, et al. A phase II study of the histone deacetylase inhibitor panobinostat (LBH589) in pretreated patients with small-cell lung cancer. J Thorac Oncol. 2013;8(8):1091–4.
Rathkopf DE, Picus J, Hussain A, et al. A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2013;72(3):537–44.
Hainsworth JD, Infante JR, Spigel DR, et al. A phase II trial of panobinostat, a histone deacetylase inhibitor, in the treatment of patients with refractory metastatic renal cell carcinoma. Cancer Invest. 2011;29(7):451–5.
Cassier PA, Lefranc A, Amela EY, et al. A phase II trial of panobinostat in patients with advanced pretreated soft tissue sarcoma. A study from the French Sarcoma Group. Br J Cancer. 2013;109(4):909–14.
Wang H, Cao Q, Dudek AZ. Phase II study of panobinostat and bortezomib in patients with pancreatic cancer progressing on gemcitabine-based therapy. Anticancer Res. 2012;32(3):1027–31.
Lee EQ, Reardon DA, Schiff D, et al. Panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma [abstract no. 2020]. In: 50th Annual Meeting of the American Society of Clinical Oncology. 2014.
Dimicoli S, Jabbour E, Borthakur G, et al. Phase II study of the histone deacetylase inhibitor panobinostat (LBH589) in patients with low or intermediate-1 risk myelodysplastic syndrome. Am J Hematol. 2012;87(1):127–9.
Platzbecker U, Al-Ali HK, Gattermann N, et al. Phase 2 study of oral panobinostat (LBH589) with or without erythropoietin in heavily transfusion-dependent IPSS low or int-1 MDS patients. Leukemia. 2014;28(3):696–8.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. K. P. Garnock-Jones is a salaried employee of Adis, Springer SBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
This profile has been extracted and modified from the Adis R&D Insight drug pipeline database. Adis R&D Insight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch.
Rights and permissions
About this article
Cite this article
Garnock-Jones, K.P. Panobinostat: First Global Approval. Drugs 75, 695–704 (2015). https://doi.org/10.1007/s40265-015-0388-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0388-8