Abstract
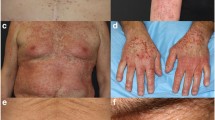
Tyrosine kinase inhibitors (TKIs) that target epidermal growth factor receptor (EGFR) have dramatically improved progression-free survival in non-small-cell lung cancer (NSCLC) patients who carry sensitizing EGFR-activating mutations and in patients with breast and pancreatic cancers. However, EGFR-TKIs are associated with significant and disabling undesirable effects that adversely impact on quality of life and compliance. These effects include dermatological reactions, diarrhoea, hepatotoxicity, stomatitis, interstitial lung disease and ocular toxicity. Each individual EGFR-TKI is also associated with additional adverse effect(s) that are not shared widely by the other members of its class. Often, these effects call for dose reduction, treatment discontinuation or pharmacotherapeutic intervention. Since dermatological effects result from on-target effects on wild-type EGFR, rash is often considered to be a biomarker of efficacy. A number of studies have reported better outcomes in patients with skin reactions compared with those without. This has led to a ‘dosing-to-rash’ strategy to optimize therapeutic outcomes. Although conceptually attractive, there is currently insufficient evidence-based support for this strategy. While skin reactions following EGFR-TKIs are believed to result from an effect on wild-type EGFR, their efficacy is related to effects on mutant variants of EGFR. It is noteworthy that newer EGFR-TKIs that spare wild-type EGFR are associated with fewer dermatological reactions. Furthermore, secondary mutations such as T790M in exon 20 often lead to development of resistance to the clinical activity and efficacy of first- and second-generation EGFR-TKIs. This has stimulated the search for later-generations of EGFR-TKIs with the ability to overcome this resistance and with greater target selectivity to spare wild-type EGFR in expectations of an improved safety profile. However, available data reviewed herein indicate that not only are these newer agents associated with the aforementioned adverse effects typical of earlier agents, but they are also susceptible to resistance due to tertiary mutations, most frequently C797S. At least three later-generation EGFR-TKIs, canertinib, naquotinib and rociletinib, have been discontinued from further development in NSCLC following concerns about their safety and risk/benefit.
Similar content being viewed by others
References
Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–43.
Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25:282–303.
Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74.
Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11:689–708.
Sheng Z, Zhang Y. The efficacy of epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer harboring wild-type epidermal growth factor receptor: a meta-analysis of 25 RCTs. Am J Clin Oncol. 2017;40:362–9.
Chung C. Tyrosine kinase inhibitors for epidermal growth factor receptor gene mutation-positive non-small cell lung cancers: an update for recent advances in therapeutics. J Oncol Pharm Pract. 2016;22:461–76.
Calvo E, Baselga J. Ethnic differences in response to epidermal growth factor receptor tyrosine kinase inhibitors. J Clin Oncol. 2006;24:2158–63.
Lee CK, Brown C, Gralla RJ, Hirsh V, Thongprasert S, Tsai CM, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595–605.
Ellis PM, Coakley N, Feld R, Kuruvilla S, Ung YC. Use of the epidermal growth factor receptor inhibitors gefitinib, erlotinib, afatinib, dacomitinib, and icotinib in the treatment of non-small-cell lung cancer: a systematic review. Curr Oncol. 2015;22:e183–215.
Tan CS, Kumarakulasinghe NB, Huang YQ, Ang YLE, Choo JR, Goh BC, et al. Third generation EGFR TKIs: current data and future directions. Mol Cancer. 2018;17:29.
Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92.
Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73.
Zhang K, Yuan Q. Current mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and updated therapy strategies in human non-small cell lung cancer. J Cancer Res Ther. 2016;12(Suppl):C131–7.
Dorantes-Heredia R, Ruiz-Morales JM, Cano-García F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl Lung Cancer Res. 2016;5:401–12.
Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: a systematic review and pooled analysis. Cancer Treat Rev. 2017;59:117–22.
Tomasello C, Baldessari C, Napolitano M, Orsi G, Grizzi G, Bertolini F, et al. Resistance to EGFR inhibitors in non-small cell lung cancer: clinical management and future perspectives. Crit Rev Oncol Hematol. 2018;123:149–61.
Lu X, Yu L, Zhang Z, Ren X, Smaill JB, Ding K. Targeting EGFRL858R/T790M and EGFRL858R/T790M/C797S resistance mutations in NSCLC: current developments in medicinal chemistry. Med Res Rev. 2018;38:1550–81.
Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29(Suppl 1):i10–9.
Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17:38.
Singh M, Jadhav HR. Targeting non-small cell lung cancer with small-molecule EGFR tyrosine kinase inhibitors. Drug Discov Today. 2018;23:745–53.
Zhang H. Three generations of epidermal growth factor receptor tyrosine kinase inhibitors developed to revolutionize the therapy of lung cancer. Drug Des Dev Ther. 2016;10:3867–72.
Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34.
Wang S, Song Y, Yan F, Liu D. Mechanisms of resistance to third-generation EGFR tyrosine kinase inhibitors. Front Med. 2016;10:383–8.
Liu Y, Li Y, Ou Q, Wu X, Wang X, Shao YW, et al. Acquired EGFR L718V mutation mediates resistance to osimertinib in non-small cell lung cancer but retains sensitivity to afatinib. Lung Cancer. 2018;118:1–5.
Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res. 2018;24:3097–107.
Mojtabavi Naeini M, Tavassoli M, Ghaedi K. Systematic bioinformatic approaches reveal novel gene expression signatures associated with acquired resistance to EGFR targeted therapy in lung cancer. Gene. 2018;667:62–9.
Fogli S, Polini B, Del Re M, Petrini I, Passaro A, Crucitta S, et al. EGFR-TKIs in non-small-cell lung cancer: focus on clinical pharmacology and mechanisms of resistance. Pharmacogenomics. 2018;19:727–40.
Food and Drug Administration. Drug-specific reviews on Drugs@FDA. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed July 2018.
European Medicines Agency. Drug-specific assessment reports and labels. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124. Accessed July 2018.
Kang SP, Ratain MJ. Inconsistent labeling of food effect for oral agents across therapeutic areas: differences between oncology and non-oncology products. Clin Cancer Res. 2010;16:4446–51.
Szmulewitz RZ, Ratain MJ. Playing Russian roulette with tyrosine kinase inhibitors. Clin Pharmacol Ther. 2013;93:242–4.
Yu G, Zheng QS, Wang DX, Zhou HH, Li GF. Drug interactions between tyrosine-kinase inhibitors and acid suppressive agents: more than meets the eye. Lancet Oncol. 2014;15:e469–70.
Waters NJ. Evaluation of drug-drug interactions for oncology therapies: in vitro-in vivo extrapolation model-based risk assessment. Br J Clin Pharmacol. 2015;79:946–58.
Gay C, Toulet D, Le Corre P. Pharmacokinetic drug-drug interactions of tyrosine kinase inhibitors: a focus on cytochrome P450, transporters, and acid suppression therapy. Hematol Oncol. 2017;35:259–80.
Peters S, Zimmermann S, Adjei AA. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: comparative pharmacokinetics and drug–drug interactions. Cancer Treat Rev. 2014;40:917–26.
Zhang L, Wu F, Lee SC, Zhao H, Zhang L. pH-dependent drug–drug interactions for weak base drugs: potential implications for new drug development. Clin Pharmacol Ther. 2014;96:266–77.
Kumarakulasinghe NB, Syn N, Soon YY, Asmat A, Zheng H, Loy EY, et al. EGFR kinase inhibitors and gastric acid suppressants in EGFR-mutant NSCLC: a retrospective database analysis of potential drug interaction. Oncotarget. 2016;7:85542–50.
Suzumura T, Kimura T, Kudoh S, Umekawa K, Nagata M, Matsuura K, et al. Reduced CYP2D6 function is associated with gefitinib-induced rash in patients with non-small cell lung cancer. BMC Cancer. 2012;12:568.
Food and Drug Administration. Label for ALUNBRIG (brigatinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208772lbl.pdf. Accessed 28 Apr 2017.
Ruan CJ, Liu DY, Jiang J, Hu P. Effect of the CYP2C19 genotype on the pharmacokinetics of icotinib in healthy male volunteers. Eur J Clin Pharmacol. 2012;68:1677–80.
Wind S, Giessmann T, Jungnik A, Brand T, Marzin K, Bertulis J, et al. Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clin Drug Investig. 2014;34:173–82.
Wind S, Schnell D, Ebner T, Freiwald M, Stopfer P. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin Pharmacokinet. 2017;56:235–50.
Wiebe S, Schnell D, Külzer R, Gansser D, Weber A, Wallenstein G, et al. Influence of renal impairment on the pharmacokinetics of afatinib: an open-label, single-dose study. Eur J Drug Metab Pharmacokinet. 2017;42:461–9.
Hu X, Han B, Gu A, Zhang Y, Jiao SC, Wang CL, et al. A single-arm, multicenter, safety-monitoring, phase IV study of icotinib in treating advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2014;86:207–12.
Zhang J, Zhan Y, Ouyang M, Qin Y, Zhou C, Chen R. Fatal interstitial lung disease associated with icotinib. J Thorac Dis. 2014;6:E267–71.
Chen X, Zhu Q, Liu Y, Liu P, Yin Y, Guo R, et al. Icotinib is an active treatment of non-small-cell lung cancer: a retrospective study. PLoS One. 2014;9:e95897.
Liu D, Zhang L, Wu Y, Jiang J, Tan F, Wang Y, et al. Clinical pharmacokinetics, safety, and preliminary efficacy evaluation of icotinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2015;89:262–7.
Xue ZX, Wen WX, Zhuang Y, Hua ZJ, Xia YN. Comparison of the efficacy of icotinib in patients with non-small-cell lung cancer according to the type of epidermal growth factor receptor mutation. Mol Clin Oncol. 2016;5:265–8.
Biaoxue R, Hua L, Wenlong G, Shuanying Y. Efficacy and safety of icotinib in treating non-small cell lung cancer: a systematic evaluation and meta-analysis based on 15 studies. Oncotarget. 2016;7:86902–13.
Park K, Lee J-S, Han J-Y, Lee KH, Kim J-H, Cho EK, et al. Efficacy and safety of BI 1482694 (HM61713), an EGFR mutant-specific inhibitor, in T790M-positive NSCLC at the recommended phase II dose. J Thorac Oncol. 2016;11(Suppl. 4S):abstract 1300.
Liao BC, Lin CC, Lee JH, Yang JC. Update on recent preclinical and clinical studies of T790M mutant-specific irreversible epidermal growth factor receptor tyrosine kinase inhibitors. J Biomed Sci. 2016;23:86.
Barnes TA, O’Kane GM, Vincent MD, Leighl NB. Third-generation tyrosine kinase inhibitors targeting epidermal growth factor receptor mutations in non-small cell lung cancer. Front Oncol. 2017;7:113.
Food and Drug Administration. Label for TARCEVA (erlotinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf. Accessed 18 Oct 2016.
Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–12.
Holcmann M, Sibilia M. Mechanisms underlying skin disorders induced by EGFR inhibitors. Mol Cell Oncol. 2015;2:e1004969.
Kozuki T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn J Clin Oncol. 2016;46:291–8.
Gutzmer R, Wollenberg A, Ugurel S, Homey B, Ganser A, Kapp A. Cutaneous side effects of new antitumor drugs: clinical features and management. Dtsch Arztebl Int. 2012;109:133–40.
Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013;36:413–26.
Charles C, Bungener C, Razavi D, Mateus C, Routier E, Lanoy E, et al. Impact of dermatologic adverse events induced by targeted therapies on quality of life. Crit Rev Oncol Hematol. 2016;101:158–68.
Wang J, Cheng X, Lu Y, Zhou B. A case report of toxic epidermal necrolysis associated with AZD-9291. Drug Des Dev Ther. 2018;12:2163–7.
Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E. Dermatologic toxicity occurring during anti-EGFR monoclonal inhibitor therapy in patients with metastatic colorectal cancer: a systematic review. Clin Colorectal Cancer. 2018;17:85–96.
Ocvirk J, Heeger S, McCloud P, Hofheinz RD. A review of the treatment options for skin rash induced by EGFR-targeted therapies: evidence from randomized clinical trials and a meta-analysis. Radiol Oncol. 2013;47:166–75.
Eriksen JG, Kaalund I, Clemmensen O, Overgaard J, Pfeiffer P. Placebo-controlled phase II study of vitamin K3 cream for the treatment of cetuximab-induced rash. Support Care Cancer. 2017;25:2179–85.
Melosky B, Anderson H, Burkes RL, Chu Q, Hao D, Ho V, et al. Pan Canadian Rash Trial: a randomized phase III trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor-tyrosine kinase inhibitor-induced skin toxicities in patients with metastatic lung cancer. J Clin Oncol. 2016;34:810–5.
Hwang IG, Kang JH, Oh SY, Lee S, Kim SH, Song KH, et al. Phase II trial of epidermal growth factor ointment for patients with erlotinib-related skin effects. Support Care Cancer. 2016;24:301–9.
Iimura Y, Shimomura H, Yasu T, Imanaka K, Ogawa R, Ito A, Suzuki K, Yamaguchi G, Kawasaki N, Konaka C. NSAIDs may prevent EGFR-TKI-related skin rash in non-small cell lung cancer patients . Int J Clin Pharmacol Ther. 2018 (epub ahead of print).
Perez-Soler R. Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer. 2006;8(Suppl 1):S7–14.
Dienstmann R, Braña I, Rodon J, Tabernero J. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist. 2011;16:1729–40.
Aranda E, Manzano JL, Rivera F, Galán M, Valladares-Ayerbes M, Pericay C, et al. Phase II open-label study of erlotinib in combination with gemcitabine in unresectable and/or metastatic adenocarcinoma of the pancreas: relationship between skin rash and survival (Pantar study). Ann Oncol. 2012;23:1919–25.
Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: a literature-based meta-analysis of 24 trials. Lung Cancer. 2012;78:8–15.
Fiala O, Pesek M, Finek J, Krejci J, Ricar J, Bortlicek Z, et al. Skin rash as useful marker of erlotinib efficacy in NSCLC and its impact on clinical practice. Neoplasma. 2013;60:26–32.
Stepanski EJ, Reyes C, Walker MS, Satram-Hoang S, Leon L, Wojtowicz-Praga S, et al. The association of rash severity with overall survival: findings from patients receiving erlotinib for pancreatic cancer in the community setting. Pancreas. 2013;42:32–6.
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan DM, et al. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2013;8:e55128.
Tiseo M, Andreoli R, Gelsomino F, Mozzoni P, Azzoni C, Bartolotti M, et al. Correlation between erlotinib pharmacokinetics, cutaneous toxicity and clinical outcomes in patients with advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2014;83:265–71.
Kudo K, Hotta K, Bessho A, Nogami N, Kozuki T, Kuyama S, et al. Development of a skin rash within the first week and the therapeutic effect in afatinib monotherapy for EGFR-mutant non-small cell lung cancer (NSCLC): Okayama Lung Cancer Study Group experience. Cancer Chemother Pharmacol. 2016;77:1005–9.
Steffens M, Paul T, Hichert V, Scholl C, von Mallek D, Stelzer C, et al. Dosing to rash?—The role of erlotinib metabolic ratio from patient serum in the search of predictive biomarkers for EGFR inhibitor-mediated skin rash. Eur J Cancer. 2016;55:131–9.
Fiala O, Hosek P, Pesek M, Finek J, Racek J, Stehlik P, et al. Serum concentration of erlotinib and its correlation with outcome and toxicity in patients with advanced-stage NSCLC. Anticancer Res. 2017;37:6469–76.
Barbu MA, Niţipir C, Voiosu T, Giurcăneanu C. Impact of dermatologic adverse reactions on QOL in oncologic patients: results from a single-center prospective study. Rom J Intern Med. 2018;56:96–101.
Wei F, Shin D, Cai X. Incidence, risk and prognostic role of anti-epidermal growth factor receptor-induced skin rash in biliary cancer: a meta-analysis. Int J Clin Oncol. 2018;23:443–51.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, for FLAURA Investigators, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
Mita AC, Papadopoulos K, de Jonge MJ, Schwartz G, Verweij J, Mita MM, et al. Erlotinib ‘dosing-to-rash’: a phase II intrapatient dose escalation and pharmacologic study of erlotinib in previously treated advanced non-small cell lung cancer. Br J Cancer. 2011;105:938–44.
Brahmer JR, Lee JW, Traynor AM, Hidalgo MM, Kolesar JM, Siegfried JM, et al. Dosing to rash: a phase II trial of the first-line erlotinib for patients with advanced non-small-cell lung cancer an Eastern Cooperative Oncology Group Study (E3503). Eur J Cancer. 2014;50:302–8.
Liao D, Yao D, Liu N, Cao L, Xiang D, Yang N, et al. Correlation of plasma erlotinib trough concentration with skin rash in Chinese NSCLC patients harboring exon 19 deletion mutation. Cancer Chemother Pharmacol. 2018;82:551–9.
Nishimura M, Hayashi M, Mizutani Y, Takenaka K, Imamura Y, Chayahara N, et al. Distribution of erlotinib in rash and normal skin in cancer patients receiving erlotinib visualized by matrix assisted laser desorption/ionization mass spectrometry imaging. Oncotarget. 2018;9:18540–7.
Hichert V, Scholl C, Steffens M, Paul T, Schumann C, Rüdiger S, et al. Predictive blood plasma biomarkers for EGFR inhibitor-induced skin rash. Oncotarget. 2017;8:35193–204.
Van Sebille YZ, Gibson RJ, Wardill HR, Bowen JM. ErbB small molecule tyrosine kinase inhibitor (TKI) induced diarrhoea: chloride secretion as a mechanistic hypothesis. Cancer Treat Rev. 2015;41:646–52.
Van Sebille YZA, Gibson RJ, Wardill HR, Ball IA, Keefe DMK, Bowen JM. Dacomitinib-induced diarrhea: targeting chloride secretion with crofelemer. Int J Cancer. 2018;142:369–80.
Melosky B, Hirsh V. Management of common toxicities in metastatic NSCLC related to anti-lung cancer therapies with EGFR-TKIs. Front Oncol. 2014;4:238.
Califano R, Tariq N, Compton S, Fitzgerald DA, Harwood CA, Lal R, et al. Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs. 2015;75:1335–48.
Parikh P, Prabhash K, Naik R, Vaid AK, Goswami C, Rajappa S, et al. Practical recommendation for rash and diarrhea management in Indian patients treated with tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Indian J Cancer. 2016;53:87–91.
Vogel WH, Paul J. Management strategies for adverse events associated with EGFR TKIs in non-small cell lung cancer. J Adv Pract Oncol. 2016;7:723–35.
Aw DC-W, Tan EH, Chin TM, Lim HL, Lee HY, Soo RA. Management of epidermal growth factor receptor tyrosine kinase inhibitor-related cutaneous and gastrointestinal toxicities. Asia Pac J Clin Oncol. 2018;14:23–31.
Hofheinz RD, Segaert S, Safont MJ, Demonty G, Prenen H. Management of adverse events during treatment of gastrointestinal cancers with epidermal growth factor inhibitors. Crit Rev Oncol Hematol. 2017;114:102–13.
Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36:491–503.
Wang J, Wu Y, Dong M, He X, Wang Z, Li J, et al. Observation of hepatotoxicity during long-term gefitinib administration in patients with non-small-cell lung cancer. Anticancer Drugs. 2016;27:245–50.
Yoshida H, Kim YH. Successful osimertinib rechallenge after severe osimertinib-induced hepatotoxicity. J Thorac Oncol. 2017;12:e61–3.
Fujiwara Y, Goto Y, Kanda S, Horinouchi H, Yamamoto N, Sakiyama N, et al. Efficacy and safety of osimertinib in a Japanese compassionate use program. Jpn J Clin Oncol. 2017;47:625–9.
Hirabayashi R, Fujimoto D, Satsuma Y, Hirabatake M, Tomii K. Successful oral desensitization with osimertinib following osimertinib-induced fever and hepatotoxicity: a case report. Investig New Drugs. 2018;36:952–4.
Yonesaka K, Suzumura T, Tsukuda H, Hasegawa Y, Ozaki T, Sugiura T, et al. Erlotinib is a well-tolerated alternate treatment for non-small cell lung cancer in cases of gefitinib-induced hepatotoxicity. Anticancer Res. 2014;34:5211–5.
Imai A, Hachiya T, Ikuyama Y, Sonehara K, Fujimori A, Shiba H, et al. Successful treatment of non-small cell lung cancer with afatinib after gefitinib-induced hepatotoxicity. Gan To Kagaku Ryoho. 2016;43:91–4.
Zenke Y, Umemura S, Sugiyama E, Kirita K, Matsumoto S, Yoh K, et al. Successful treatment with afatinib after grade 3 hepatotoxicity induced by both gefitinib and erlotinib in EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2016;99:1–3.
Ueda H, Hayashi H, Kudo K, Takeda M, Nakagawa K. Successful treatment with afatinib after gefitinib- and erlotinib-induced hepatotoxicity. Investig New Drugs. 2016;34:797–9.
Shah RR. Tyrosine kinase inhibitor-induced interstitial lung disease: clinical features, diagnostic challenges, and therapeutic dilemmas. Drug Saf. 2016;39:1073–91.
Sakurada T, Kakiuchi S, Tajima S, Horinouchi Y, Okada N, Nishisako H, et al. Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother. 2015;49:398–404.
Miyauchi E, Ichinose M, Inoue A. Successful osimertinib rechallenge in a patient with T790M-mutant non-small cell lung cancer after osimertinib-induced interstitial lung disease. J Thorac Oncol. 2017;12:e59–61.
Satoh S, Shiroyama T, Tamiya M, Nasu S, Tanaka A, Morita S, et al. Successful osimertinib rechallenge after osimertinib-induced pneumonitis in a patient with lung adenocarcinoma. Respir Med Case Rep. 2017;23:68–70.
Kiriu T, Tamura D, Tachihara M, Sekiya R, Hazama D, Katsurada M, et al. Successful osimertinib rechallenge with steroid therapy after osimertinib-induced interstitial lung disease. Intern Med. 2018;57:91–5.
Agustoni F, Platania M, Vitali M, Zilembo N, Haspinger E, Sinno V, et al. Emerging toxicities in the treatment of non-small cell lung cancer: ocular disorders. Cancer Treat Rev. 2014;40:197–203.
Saint-Jean A, Sainz de la Maza M, Morral M, Torras J, Quintana R, Molina JJ, et al. Ocular adverse events of systemic inhibitors of the epidermal growth factor receptor: report of 5 cases. Ophthalmology. 2012;119:1798–802.
Sun P, Long J, Chen P, He Q, Gao X, Li S. Rapid onset of conjunctivitis associated with overdosing of erlotinib. J Clin Pharm Ther. 2018;43:296–8.
Todokoro D, Itakura H, Ibe T, Kishi S. Anterior uveitis caused by ocular side effects of afatinib: a case report. Case Rep Ophthalmol. 2016;7:74–8.
Shah DR, Dholakia S, Shah RR. Effect of tyrosine kinase inhibitors on wound healing and tissue repair: implications for surgery in cancer patients. Drug Saf. 2014;37:135–49.
Shah RR. Hyperglycaemia induced by novel anticancer agents: an undesirable complication or a potential therapeutic opportunity? Drug Saf. 2017;40:211–28.
Shah RR, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf. 2013;36:295–316.
Shah RR, Morganroth J. Update on cardiovascular safety of tyrosine kinase inhibitors: with a special focus on QT interval, left ventricular dysfunction and overall risk/benefit. Drug Saf. 2015;38:693–710.
Patel H, Pawara R, Ansari A, Surana S. Recent updates on third generation EGFR inhibitors and emergence of fourth generation EGFR inhibitors to combat C797S resistance. Eur J Med Chem. 2017;142:32–47.
Russo A, Franchina T, Ricciardi GRR, Smiroldo V, Picciotto M, Zanghì M, et al. Third generation EGFR TKIs in EGFR-mutated NSCLC: where are we now and where are we going. Crit Rev Oncol Hematol. 2017;117:38–47.
Yu HA, Tian SK, Drilon AE, Borsu L, Riely GJ, Arcila ME, et al. Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol. 2015;1:982–4.
Lee J, Shim JH, Park WY, Kim HK, Sun JM, Lee SH, et al. Rare mechanism of acquired resistance to osimertinib in Korean patients with EGFR-mutated non-small cell lung cancer. Cancer Res Treat. 2018 (epub ahead of print).
Song HN, Jung KS, Yoo KH, Cho J, Lee JY, Lim SH, et al. Acquired C797S mutation upon treatment with a T790M-specific third-generation EGFR inhibitor (HM61713) in non-small cell lung cancer. J Thorac Oncol. 2016;11:e45–7.
Zhao P, Yao MY, Zhu SJ, Chen JY, Yun CH. Crystal structure of EGFR T790M/C797S/V948R in complex with EAI045. Biochem Biophys Res Commun. 2018;502:332–7.
Nemunaitis J, Eiseman I, Cunningham C, Senzer N, Williams A, Lenehan PF, et al. Phase 1 clinical and pharmacokinetics evaluation of oral CI-1033 in patients with refractory cancer. Clin Cancer Res. 2005;11:3846–53.
Jänne PA, von Pawel J, Cohen RB, Crino L, Butts CA, Olson SS, et al. Multicenter, randomized, phase II trial of CI-1033, an irreversible pan-ERBB inhibitor, for previously treated advanced non-small cell lung cancer. J Clin Oncol. 2007;25:3936–44.
Zinner RG, Nemunaitis J, Eiseman I, Shin HJ, Olson SC, Christensen J, et al. Phase I clinical and pharmacodynamic evaluation of oral CI-1033 in patients with refractory cancer. Clin Cancer Res. 2007;13:3006–14.
Rixe O, Franco SX, Yardley DA, Johnston SR, Martin M, Arun BK, et al. A randomized, phase II, dose-finding study of the pan-ErbB receptor tyrosine-kinase inhibitor CI-1033 in patients with pretreated metastatic breast cancer. Cancer Chemother Pharmacol. 2009;64:1139–48.
Han JY, Lee KH, Kim SW, Min YJ, Cho E, Lee Y, et al. A phase II study of poziotinib in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma who have acquired resistance to EGFR-tyrosine kinase inhibitors. Cancer Res Treat. 2017;49:10–9.
Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2017;35:3105–12.
Wu YL, Zhou Q, Liu X, Zhang L, Zhou J, Wu L, et al. Phase I/II study of AC0010, mutant-selective EGFR inhibitor, in non-small cell lung cancer (NSCLC) patients with EGFR T790M mutation. J Thorac Oncol. 2017;12:S437–8 (abstract MA.16.06).
Ma Y, Zheng X, Zhao H, Fang W, Zhang Y, Ge J, et al. First-in-human phase I study of AC0010, a mutant-selective EGFR inhibitor in non–small cell lung cancer: Safety, efficacy, and potential mechanism of resistance. J Thorac Oncol. 2018;13:968–77.
Goto Y, Nokihara H, Murakami H, Shimizu T, Seto T, Krivoshik AP, et al. ASP8273, a mutant-selective irreversible EGFR inhibitor in patients (pts) with NSCLC harboring EGFR activating mutations: preliminary results of first-in-human phase I study in Japan. J Clin Oncol. 2015;33(15 Suppl):abstract 8014.
Yu HA, Spira A, Horn L, Weiss J, West H, Giaccone G, et al. A phase I, dose escalation study of oral ASP8273 in patients with non-small cell lung cancers with epidermal growth factor receptor mutations. Clin Cancer Res. 2017;23:7467–73.
Tan DSW, Seto T, Leighl NB, Riely GJ, Sequist LV, Felip E, et al. First-in-human phase I study of EGF816, a third generation, mutant-selective EGFR tyrosine kinase inhibitor, in advanced non-small cell lung cancer (NSCLC) harboring T790M. J Clin Oncol. 2015;33(15 Suppl):abstract 8013.
Kim DW, Tan DSW, Aix SP, Sequist LV, Smit EF, Hida T. Preliminary Phase II results of a multicenter, open-label study of nazartinib (EGF816) in adult patients with treatment-naïve EGFR-mutant non-small cell lung cancer (NSCLC). J Clin Oncol. 2018;36(15 Suppl):abstract 9094.
Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–9.
Goldman JW, Mendenhall MA, Rettinger SR. Hyperglycemia associated with targeted oncologic treatment: mechanisms and management. Oncologist. 2016;21:1326–36.
Piotrowska Z, Liu E, Varga A, Thakur M, Narayanan V, Liu SV, et al. Rociletinib-associated cataracts in EGFR-mutant NSCLC. Ann Oncol. 2016;27(Suppl 6):416–54 (poster 1239P).
Husain H, Martins RG, Goldberg SB, Senico P, Ma W, Masters J, et al. First-in-human phase I study of PF-06747775, a third-generation mutant selective EGFR tyrosine kinase inhibitor (TKI) in metastatic EGFR mutant NSCLC after progression on a first-line EGFR TKI. Ann Oncol. 2017;28(Suppl 5):485–6 (poster 1385P).
Liu E, Kopani K. Rapidly progressive cataract formation associated with non-small-cell lung cancer therapy. J Cataract Refract Surg. 2016;42:1838–40.
Rao AC, Tiuseco K, Yun S, White A. Beware the TIGER-X’s stripes: rapid cataract formation in patients taking rociletinib. Clin Exp Ophthalmol. 2017;45:548–9.
Schwentner I, Schmutzhard J, Glueckert R, Charitidi K, Falkeis C, Sergi C, et al. Epidermal growth factor receptor expression in human fetal cochlea with Turner syndrome. Otol Neurotol. 2009;30:858–63.
Tang J, Qian Y, Li H, Kopecky BJ, Ding D, Ou HC, et al. Canertinib induces ototoxicity in three preclinical models. Hear Res. 2015;328:59–66.
Van Der Steen N, Caparello C, Rolfo C, Pauwels P, Peters GJ, Giovannetti E. New developments in the management of non-small-cell lung cancer, focus on rociletinib: what went wrong? Onco Targets Ther. 2016;9:6065–74.
Wang S, Song Y, Liu D. EAI045: the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett. 2017;385:51–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This is a review of data in the public domain and the authors declare compliance with all ethical standards.
Funding
No sources of funding were used to assist in the preparation of this review.
Conflict of interest
Rashmi Shah (RS) and Devron Shah (DS) have no conflicts of interest that are relevant to the content of this review and have not received any financial support for writing it. RS was formerly a Senior Clinical Assessor at the Medicines and Healthcare products Regulatory Agency (MHRA), London, UK, and now provides expert consultancy services to a number of pharmaceutical companies. DS is a Registrar in Respiratory Medicine in Hull, Yorkshire, UK.
Contributions for authorship
RS conceived the topic and DS assisted with the literature search. RS prepared the first draft and DS revised the first and subsequent drafts. Both RS and DS approved the final version.
Additional information
Part of a theme issue on “Safety of Novel Anticancer Therapies: Future Perspectives”. Guest Editors: Rashmi R. Shah, Giuseppe Curigliano.
Rights and permissions
About this article
Cite this article
Shah, R.R., Shah, D.R. Safety and Tolerability of Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors in Oncology. Drug Saf 42, 181–198 (2019). https://doi.org/10.1007/s40264-018-0772-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0772-x