Abstract
Background and Objective
Afatinib is a potent, irreversible, ErbB family blocker in clinical development for the treatment of advanced non-small cell lung cancer, metastatic head and neck cancer, and other solid tumours. As afatinib is a substrate for the P-glycoprotein (P-gp) pump transporter the three studies presented here investigated the pharmacokinetics of afatinib in the presence of a potent inhibitor (ritonavir) or inducer [rifampicin (rifampin)] of P-gp.
Methods
We conducted phase I, open-label, single-centre studies in healthy male volunteers who received a single once-daily oral dose of afatinib (20 or 40 mg) together with either ritonavir or rifampicin; two studies had a randomised, two- and three-way crossover design and the third was a non-randomised, two-period sequential study.
Results
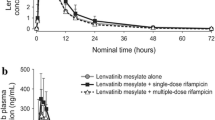
When afatinib 20 mg was administered 1 h after ritonavir, afatinib geometric mean (gMean) maximum plasma concentration (C max) and area under the plasma concentration–time curve from time zero to infinity (AUC∞) increased by 38.5 and 47.6 %, respectively. Coadministration of ritonavir either simultaneously or 6 h later than afatinib 40 mg resulted in minimal increase in the afatinib gMean C max and AUC∞ (4.1 and 18.6 % for simultaneous administration with AUC∞ not completely within the bioequivalence limits; 5.1 and 10.8 % for timed administration within the bioequivalence limits). Administration of afatinib 40 mg in the presence of rifampicin led to reduction in C max and AUC∞ by 21.6 and 33.8 %, respectively. In all studies, P-gp modulation mainly affected the extent of absorption of afatinib; there was no change in the terminal elimination half-life. The overall safety profile of afatinib was acceptable.
Conclusion
Coadministration of potent P-gp modulators had no clinically relevant effect on afatinib exposure. Effects of potent P-gp inhibitors were minimal at higher afatinib doses and can be readily managed by the timing of concomitant therapy. As afatinib is not a relevant modulator or substrate of cytochrome P450 enzymes, the drug–drug interaction potential is considered to be low.






Similar content being viewed by others
References
Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–11.
Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343:342–50.
Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34.
Wu YL, Zhou C, Hu CP, et al. LUX-Lung 6: a randomized, open-label, phase III study of afatinib (A) versus gemcitabine/cisplatin (GC) as first-line treatment for Asian patients (pts) with EGFR mutation-positive (EGFR M+) advanced adenocarcinoma of the lung. J Clin Oncol 2013;31(Suppl):abstract 8016.
Cohen EEW, Fayette J, Cupissol D, et al. A randomized, open-label, phase II study of afatinib (BIBW 2992) versus cetuximab in recurrent or metastatic squamous cell carcinoma of the head and neck: final data [abstract no. PP101]. Eur Arch Otorhinolaryngol. 2012;269:1374.
Gilotrif™ (afatinib) tablet for oral use: US prescribing information. Ridgefield: Boehringer Ingelheim Pharmaceuticals, Inc.; 2013 Oct. http://www.gilotrif.com/. Accessed 21 Nov 2013.
Wind S, Schmid M, Erhardt J, et al. Pharmacokinetics of afatinib, a selective irreversible ErbB family blocker, in patients with advanced solid tumours. Clin Pharmacokinet. 2013;52:1101–9.
Stopfer P, Marzin K, Narjes H, et al. Afatinib pharmacokinetics and metabolism after oral administration to healthy male volunteers. Cancer Chemother Pharmacol. 2012;69:1051–61.
Lin JH, Yamazaki M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet. 2003;42:59–98.
Mizuno N, Niwa T, Yotsumoto Y, et al. Impact of drug transporter studies on drug discovery and development. Pharmacol Rev. 2003;55:425–61.
Marzolini C, Paus E, Buclin T, et al. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33.
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) assessment report for Giotrif (afatinib). 16 October 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002280/WC500152394.pdf. Accessed 20 Nov 2013.
Zhang L, Strong JM, Qiu W, et al. Scientific perspectives on drug transporters and their role in drug interactions. Mol Pharm. 2006;3:62–9.
Backman JT, Olkkola KT, Ojala M, et al. Concentrations and effects of oral midazolam are greatly reduced in patients treated with carbamazepine or phenytoin. Epilepsia. 1996;37:253–7.
Backman JT, Kivisto KT, Olkkola KT, et al. The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol. 1998;54:53–8.
FDA Center for Drug Evaluation and Research (CDER). Guidance for industry. Drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations. US FDA draft guidance, Feb 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf Accessed 27 Aug 2013.
European Medicines Agency. Committee for Human Medicinal Products (CHMP). Guideline on the investigation of drug interactions, 21 June 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf. Accessed 27 Aug 2013.
Norvir® (ritonavir) tablet for oral use: US prescribing information. North Chicago: AbbVie Inc.; 2013 Nov. http://www.norvir.com/. Accessed 21 Nov 2013.
Hsu A, Granneman GR, Bertz RJ. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35:275–91.
Niemi M, Backman JT, Fromm MF, et al. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50.
Galteau MM, Shamsa F. Urinary 6beta-hydroxycortisol: a validated test for evaluating drug induction or drug inhibition mediated through CYP3A in humans and in animals. Eur J Clin Pharmacol. 2003;59:713–33.
Tran JQ, Kovacs SJ, McIntosh TS, et al. Morning spot and 24-hour urinary 6 beta-hydroxycortisol to cortisol ratios: intraindividual variability and correlation under basal conditions and conditions of CYP 3A4 induction. J Clin Pharmacol. 1999;39:487–94.
Härtter S, Sennewald R, Nehmiz G, et al. Oral bioavailability of dabigatran etexilate (Pradaxa®) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75:1053–62.
International Transporter Consortium, Giacomini KM, Huang SM, et al. Membrane transporters in drug development. Nat Rev Drug Discov 2010;9:215–36.
Gupta A, Zhang Y, Unadkat JD, et al. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther. 2004;310:334–41.
Tachibana T, Kato M, Watanabe T, et al. Method for predicting the risk of drug–drug interactions involving inhibition of intestinal CYP3A4 and P-glycoprotein. Xenobiotica. 2009;39:430–43.
Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–23.
Acknowledgments
These studies were sponsored by Boehringer Ingelheim Pharma GmbH & Co. KG, Germany. The studies were conducted at the Human Pharmacology Centre, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany (principal investigator, Mario Iovino). Boehringer Ingelheim was responsible for the design and conduct of all of the studies, and the collection and management of the data. The authors were responsible for the analysis and interpretation of the data and the preparation of the manuscript. All authors are employees of Boehringer Ingelheim and were fully responsible for all content and editorial decisions, and were involved at all stages of the manuscript development.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wind, S., Giessmann, T., Jungnik, A. et al. Pharmacokinetic Drug Interactions of Afatinib with Rifampicin and Ritonavir. Clin Drug Investig 34, 173–182 (2014). https://doi.org/10.1007/s40261-013-0161-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0161-2