Abstract
Introduction
H1 antihistamines are used for the treatment of nausea and vomiting during pregnancy as well as the symptomatic relief of asthma, urticaria, allergy, and the common cold. Although they are overall felt to be safe during pregnancy, recently several studies have challenged this assumption, as millions of women are exposed to them in the first trimester.
Methods
Following the guidelines of PRISMA, a systematic review was performed to retrieve all published articles involving H1-antihistamine exposure during pregnancy. Electronic databases including PubMed and EMBASE were searched for possibly relevant articles published in any language up to December 2015.
Results
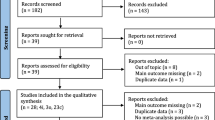
After removing duplicate publications, and excluding animal studies and studies on drug effectiveness, 342 articles were reviewed in detail and 37 studies fulfilled the inclusion criteria for the meta-analysis. In cohort studies, the risk of major malformation in the offspring of women exposed to H1 antihistamines was not higher than that of the control population (OR 1.07; 95% CI 0.98–1.16). The Q-statistic for heterogeneity of effects was not significant (p > 0.05, I 2 < 25%) and there was no evidence of publication bias. Similar results were achieved with case–control studies (OR 1.05; 95% CI 0.90–1.23). Similarly, H1 antihistamines were not associated with more spontaneous abortions (OR 1.00; 95% CI 0.83–1.20), prematurity (OR 0.96; 95% CI 0.76–1.20), stillbirth (OR 1.23; 95% CI 0.48–3.18) or low birth weight (OR 1.20; 95% CI 0.63–2.29).
Conclusions
Based on our meta-analyses, which included a large number of studies, H1 antihistamines are not associated with an increased risk of major malformation or other adverse fetal outcomes. This study provides important information to both pregnant women and their healthcare providers regarding the safety and risk of H1 antihistamine use during this sensitive time.










Similar content being viewed by others
References
Stephansson O, Granath F, Svensson T, Haglund B, Ekbom A, Kieler H. Drug use during pregnancy in Sweden - assessed by the Prescribed Drug Register and the Medical Birth Register. Clin Epidemiol. 2011;3:43–50.
Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193:771–7.
Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract. 2013;1(6):666–74.
Woolhouse M. Complementary medicine for pregnancy complications. Aust Fam Physician. 2006;35(9):695.
Bousquet J, Godard P, Michel FB. Antihistamines in the treatment of asthma. Eur Respir J. 1992;5(9):1137–42.
Soll AH, Walsh JH. Regulation of gastric acid secretion. Annu Rev Physiol. 1979;41:35–53.
Aldridge TD, Hartmann KE, Michels KA, Velez Edwards DR. First-trimester antihistamine exposure and risk of spontaneous abortion or preterm birth. Pharmacoepidemiol Drug Saf. 2014;23(10):1043–50.
Shields KE, Wiholm BE, Hostelley LS, Striano LF, Arena SR, Sharrar RG. Monitoring outcomes of pregnancy following drug exposure: a company-based pregnancy registry program. Drug Saf. 2004;27:353–67.
Etwel F, Hutson JR, Madadi P, Gareri J, Koren G. Fetal and perinatal exposure to drugs and chemicals: novel biomarkers of risk. Annu Rev Pharmacol Toxicol. 2014;54:295–315.
Seto A, Einarson T, Koren G. Pregnancy outcome following first trimester exposure to antihistamines: meta-analysis. Am J Perinatol. 1997;14(3):119–24.
Chin JW, Gregor S, Persaud N. Re-analysis of safety data supporting doxylamine use for nausea and vomiting of pregnancy. Am J Perinatol. 2014;31(8):701–10.
McKeigue PM, Lamm SH, Linn S, Kutcher JS. Bendectin and birth defects: I. A meta-analysis of the epidemiologic studies. Teratology. 1994;50(1):27–37.
MacMahon B. More on Bendectin. JAMA. 1981;246(4):371–2.
Einarson TR, Leeder JS, Koren G. A method for meta-analysis of epidemiological studies. Drug Intell Clin Pharm. 1988;22(10):813–24.
Schwarz EB, Moretti ME, Nayak S, Koren G. Risk of hypospadias in offspring of women using loratadine during pregnancy: a systematic review and meta analysis. Drug Saf. 2008;31(9):775–88.
Etwel F, Djokanovic N, Moretti ME, Boskovic R, Martinovic J, Koren G. The fetal safety of cetirizine: an observational cohort study and meta-analysis. J Obstet Gynaecol. 2014;34(5):392–9.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–80.
Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA, National Birth Defects Prevention Study. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67(3):193–201.
De Neubourg D, van Duijnhoven NT, Nelen WL, D’Hooghe TM. Dutch translation of the ICMART-WHO revised glossary on ART terminology. Gynecol Obstet Invest. 2012;74(3):233–48.
Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://www.cochrane-handbook.org.
Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
General Practitioner Research Group. General practitioner clinical trials: drugs in pregnancy survey. Practitioner. 1963;191:775–80.
Mellin G, Katzenstein M. Meclozine and fetal abnormalities. Lancet. 1963;26:222–3.
Bunde CA, Bowles DM. A technique for controlled survey of case records. Curr Ther Res Clin Exp. 1963;5:245–8.
Yerushalmy J, Milkovich L. Evaluation of the teratogenic effect of meclizine in man. Am J Obstet Gynecol. 1965;93(4):553–62.
Erez S, Schifrin BS, Dirim O. Double-blind evaluation of hydroxyzine as an antiemetic in pregnancy. J Reprod Med. 1971;7(1):35–7.
Milkovich L,vandenBerg BJ. An evaluation of the teratogenicity of certain antinauseant drugs. Am J Obstet Gynecol. 1976;125(2):244–248.
Kullander S, Källén B. A prospective study of drugs and pregnancy. II. Anti-emetic drugs. Acta Obstet Gynecol Scand. 1976;55(2):105–11.
Heinonen OP, Slone D, Shapiro S. Birth defects and drugs in pregnancy. Littleton: PSG Publishing; 1977.
Newman NM, Correy JF, Dudgeon GI. A survey of congenital abnormalities and drugs in a private practice. Aust N Z J Gynaecol. 1977;17:156–9.
Shapiro S, Kaufman DW, Rosenberg L, Slone D, Monson RR, Siskind V, Heinonen OP. Meclizine in pregnancy in relation to congenital malformations. Br Med J. 1978;1(6111):483.
Smithells RW, Sheppard S. Teratogenicity testing in humans: a method demonstrating safety of bendectin. Teratology. 1978;17(1):3–135.
Jick H, Holmes LB, Hunter JR, Madsen S, Stergachis A. First trimester drug use and congenital disorders. JAMA. 1981;246(4):343–6.
Fleming DM, Knox JDE, Crombie DL. Debendox in early pregnancy and fetal malformation. Br Med J (Clin Res Ed). 1981;283(6284):99–101.
Gibson GT, Colley DP, McMichael AJ, Hartshorne JM. Congenital anomalies in relation to the use of doxylamine/dicyclomine and other antenatal factors: an ongoing prospective study. Med J Aust. 1981;1(8):410–4.
Michaelis J, Michaelis H, Glück E, Koller S. Prospective study of suspected associations between certain drugs administered during early pregnancy and congenital malformations. Teratology. 1983;27(1):57–64.
Seto A, Einarson T, Koren G. Evaluation of brompheniramine safety in pregnancy. Reprod Toxicol. 1993;7(4):393–5.
Pastuszak A, Schick B, D’Alimonte D, Donnenfeld A, Koren G. The safety of astemizole in pregnancy. J Allergy Clin Immunol. 1996;98(4):748–50.
Einarson A, Bailey B, Jung G, Spizzirri D, Baillie M, Koren G. Prospective controlled study of hydroxyzine and cetirizine in pregnancy. Ann Allergy Asthma Immunol. 1997;78(2):183–6.
Schatz M, Petitti D. Antihistamines and pregnancy. Ann Allergy Asthma Immunol. 1997;78(2):157–9.
Wilton LV, Pearce GL, Martin RM, Mackay FJ, Mann RD. The outcomes of pregnancy in women exposed to newly marketed drugs in general practice in England. Br J Obstet Gynaecol. 1998;105(8):882–9.
Loebstein R, Lalkin A, Addis A, Costa A, Lalkin I, Bonati M, Koren G. Pregnancy outcome after gestational exposure to terfenadine: a multicenter, prospective controlled study. J Allergy Clin Immunol. 1999;104(5):953–6.
Källén B. Use of antihistamine drugs in early pregnancy and delivery outcome. J Matern Fetal Neonatal Med. 2002;11(3):146–52.
Källén B, Mottet I. Delivery outcome after the use of meclozine in early pregnancy. Eur J Epidemiol. 2003;18(7):665–9.
Bsat FA, Hoffman DE, Seubert DE. Comparison of three outpatient regimens in the management of nausea and vomiting in pregnancy. J Perinatol. 2003;23(7):531–5.
Diav-Citrin O, Shechtman S, Aharonovich A, Moerman L, Arnon J, Wajnberg R, Ornoy A. Pregnancy outcome after gestational exposure to loratadine or antihistamines: a prospective controlled cohort study. J Allergy Clin Immunol. 2003;111(6):1239–43.
Moretti ME, Caprara D, Coutinho CJ, Bar-Oz B, Berkovitch M, Addis A, Jovanovski E, Schüler-Faccini L, Koren G. Fetal safety of loratadine use in the first trimester of pregnancy: a multicenter study. J Allergy Clin Immunol. 2003;111(3):479–83.
Paulus W, Schloemp S, Sterzik K, Stoz F. Pregnancy outcome after exposure to cetirizine/levocetirizine in the first trimester-a prospective controlled study. Reprod Toxicol. 2004;19(2):258 [Abstract].
Boskovic R, Rudic N, Danieliewska-Nikiel B, Navioz Y, Koren G. Is lack of morning sickness teratogenic? A prospective controlled study. Birth Defects Res A Clin Mol Teratol. 2004;70(8):528–30.
Weber-Schoendorfer C, Schaefer C. The safety of cetirizine during pregnancy. A prospective observational cohort study. Reprod Toxicol. 2008;26(1):19–23.
Ashkenazi-Hoffnung L, Merlob P, Stahl B, Klinger G. Evaluation of the efficacy and safety of bi-daily combination therapy with pyridoxine and doxylamine for nausea and vomiting of pregnancy. Isr Med Assoc J. 2013;15(1):23–6.
Eskenazi B, Bracken MB. Bendectin (Debendox) as a risk factor for pyloric stenosis. Am J Obstet Gynecol. 1982;144(8):919–24.
Czeizel AE, Vargha P. A case-control study of congenital abnormality and dimenhydrinate usage during pregnancy. Arch Gynecol Obstet. 2005;271(2):113–8.
Gilboa SM, Strickland MJ, Olshan AF, Werler MM, Correa A, National Birth Defects Prevention Study. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol. 2009;85(2):137–50.
Petik D, Acs N, Bánhidy F, Czeizel AE. A study of the potential teratogenic effect of large doses of promethazine used for a suicide attempt by 32 pregnant women. Toxicol Ind Health. 2008;24(1–2):87–96.
Little BB, Snell LM, Breckenridge JD, Knoll KA, Klein VR, Gilstrap LC. Effects of T’s and blues abuse on pregnancy outcome and infant health status. Am J Perinatol. 1990;7(4):359–62.
Chasnoff IJ, Hatcher R, Burns WJ, Schnoll SH. Pentazocine and tripelennamine (‘T’s and blue’s’): effects on the fetus and neonate. Dev Pharmacol Ther. 1983;6(3):162–9.
Koren G, Madjunkova S, Maltepe C. The protective effects of nausea and vomiting of pregnancy against adverse fetal outcome–a systematic review. Reprod Toxicol. 2014;47:77–80.
Matsuyama K, Ichikawa T, Nitta Y, Ikoma Y, Ishimura K, Horio S, Fukui H. Localized expression of histamine H1 receptors in syncytiotrophoblast cells of human placenta. J Pharmacol Sci. 2006;102(3):331–7.
Zohdi V, Sutherland MR, Lim K, Gubhaju L, Zimanyi MA, Black MJ. Low birth weight due to intrauterine growth restriction and/or preterm birth: effects on nephron number and long-term renal health. Int J Nephrol. 2012;2012:136942.
Ornoy A, Mastroiacovo P. More on data from teratogen information systems (TIS). Teratology. 2000;61(5):327–8.
Liu S, Wen SW, Demissie K, Marcoux S, Kramer MS. Maternal asthma and pregnancy outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2001;184(2):90–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This systematic review of published manuscripts meets the criteria for ethical standards.
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Fatma Etwel, Lauren H. Faught, Michael J. Rieder and Gideon Koren have no conflicts of interest that are directly relevant to the content of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Etwel, F., Faught, L.H., Rieder, M.J. et al. The Risk of Adverse Pregnancy Outcome After First Trimester Exposure to H1 Antihistamines: A Systematic Review and Meta-Analysis. Drug Saf 40, 121–132 (2017). https://doi.org/10.1007/s40264-016-0479-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0479-9



