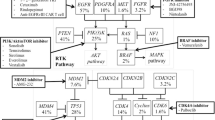
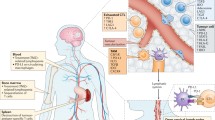
Abstract
Glioblastoma is the most frequent malignant brain tumor and is characterized by poor prognosis, increased invasiveness, and high recurrence rates. Standard treatment for glioblastoma includes maximal safe surgical resection, radiation, and chemotherapy with temozolomide. Despite treatment advances, only 15–20% of glioblastoma patients survive to 5 years, and no therapies have demonstrated a durable survival benefit in recurrent disease. In the last 10 years, significant advances in knowledge of the biology and molecular pathology of the malignancy have opened the way to new treatment options. Clinical management of patients (pseudo-progressions, side effects of therapies, best supportive care, centralization in expertise care centers) has improved. In brain tumors, such as in other solid tumors, we have entered an era of immune-oncology. Immunotherapy seems to have an acceptable safety and tolerability profile in the recurrent setting and is under investigation in clinical trials in newly diagnosed glioblastoma patients. This review focuses on novel targeted therapies recently developed for the management of newly diagnosed and recurrent glioblastomas.
Similar content being viewed by others
References
Levin VA, et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18(2):321–4.
Prados MD. Future directions in the treatment of malignant gliomas with temozolomide. Semin Oncol. 2000;27(3 Suppl 6):41–6.
Prados MD, et al. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: A retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999;17(11):3389–95.
Westphal M, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88.
Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Chinot OL, Wick W, Cloughesy T. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2049.
Sandmann T, et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33(25):2735–44.
Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2048–49.
Herrlinger U, et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol. 2016;34(14):1611–9.
Stupp R, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(16):2712–8.
Stupp R, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–8.
Nabors LB, et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol. 2015;17(5):708–17.
Wick W, et al. Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082). Clin Cancer Res. 2016;22(19):4797–806.
Gilbert MR, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–91.
Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003.
Chen R, Cohen AL, Colman H. Targeted therapeutics in patients with high-grade gliomas: past, present, and future. Curr Treat Options Oncol. 2016;17(8):42.
Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep. 2013;13(5):345.
Louis DN, et al. The World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20.
Brandes AA, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–7.
Wick K, et al. Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol. 2016;18(10):1434–41.
Brandes AA, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–9.
Schiffgens S, et al. Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget. 2016;7(34):55169–80.
Regelsberger J, Hagel C, Emami P, Ries T, Heese O, Westphal M. Risk analysis of severe myelotoxicity with temozolomide: the effects of clinical and genetic factors. Neuro Oncol. 2009;11(6):825–32. doi:10.1215/15228517-2008-120.
Jen JF, et al. Population pharmacokinetics of temozolomide in cancer patients. Pharm Res. 2000;17(10):1284–9.
Keime-Guibert F, Chinot OL, Taillanndier L, et al. Phase III study comparing radiotherapy with supportive care in older patients with newly diagnosed anaplastic astrocyotmas (AA) og glioblastoma multiforme (GBM): an ANOCEF group trial. Neuro Oncol. 2005;7(3):349.
Roa W, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–8.
Wick W, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–15.
Malmström A, Grønberg BH, Marosi C, Nordic Clinical Brain Tumour Study Group (NCBTSG), et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–26.
JR Perry, et al. A randomized phase III study of temozolomide and short-course radiation vs. short-course radiation alone in the treatment of newly diagnosed glioblastoma in elderly patients. CCTG CE.6, EORTC 26062-22061, TROG 08.02. ASCO 2016.
Brandes AA, et al. Pattern of care and effectiveness of treatment for glioblastoma patients in the real world: results from a prospective population-based registry. Could survival differ in a high-volume center? Neurooncol Pract. 2014;1(4):166–71.
Holland KD. Efficacy, pharmacology, and adverse effects of antiepileptic drugs. Neurol Clin. 2001;19(2):313–45.
Oberndorfer S, et al. P450 enzyme inducing and non-enzyme inducing antiepileptics in glioblastoma patients treated with standard chemotherapy. J Neurooncol. 2005;72(3):255–60.
Wen PY, Marks PW. Medical management of patients with brain tumors. Curr Opin Oncol. 2002;14(3):299–307.
Brandes AA, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer. 1997;33(10):1592–6.
Dietrich J, et al. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4(2):233–42.
Brandes AA, et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemoth Pharmacol. 2009;64(4):769–75.
Brada M, Stenning S, Gabe R, et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol. 2010;28:4601–8.
Brandes AA, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO). Br J Cancer. 2006;95(9):1155–60.
Wick A, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25:3357–61.
Perry RJ, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–7.
Taal W, et al. Dose dense 1 week on/1 week off temozolomide in recurrent glioma: a retrospective study. J Neurooncol. 2012;108(1):195–200.
Norden AD, et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol. 2013;15(7):930–5. doi:10.1093/neuonc/not040 (Epub 2013 Apr 3).
Omuro A, et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol. 2013;15(2):242–50. doi:10.1093/neuonc/nos295 (Epub 2012 Dec 14).
Taal W, et al. A randomized phase II study of bevacizumab versus bevacizuma bplus lomustine versus lomustine single agent in recurrent glioblastoma: the Dutch BELOB study. J Clin Oncol. 2013;31(suppl; abstr 2001).
Brandes AA, Finocchiaro G, Zagonel V, et al. AVAREG: a phase II, randomized, non comparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro Oncol. 2016;18(9):1304–12.
Brandes AA, et al. Early tumour shrinkage as a survival predictor in patients with recurrent glioblastoma treated with bevacizumab in the AVAREG randomized phase II study. Oncotarget. 2017;. doi:10.18632/Oncotarget.15735.
van den Bent M, et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine versus lomustine in patients with first progression of a glioblastoma. Neuro Oncol. 2016;18(suppl_4):iv1–iv2. doi:10.1093/neuonc/now188.002
Gil-Gil Miguel J, Mesia C, Rey M, et al. Bevacizumab for the treatment of glioblastoma. Clin Med Insights Oncol. 2013;7:123–35.
Glas M, et al. Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol. 2009;27(8):1257–61.
Herrlinger U, et al. Phase II trial of lomustine plus temozolomide chemotherapy in addition to radiotherapy in newly diagnosed glioblastoma: UKT-03. J Clin Oncol. 2006;24(27):4412–7.
Phase III trial of lomustine/temozolomide combination therapy vs. standard therapy for newly diagnosed MGMT-methylated glioblastoma patients (CeTeG)—NCT01149109-2009-011252-22 (EudraCT Number).
Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–202.
Stupp R, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–43.
Mittal S, et al. Alternating electric tumor treating fields for treatment of glioblastoma: rationale, preclinical, and clinical studies. J Neurosurg. 2017;. doi:10.3171/2016.9.JNS16452.
Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–75.
Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–60.
Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54.
Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41.
Carson MJ, Doose JM, Melchior B, et al. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65.
Muller C, Holtschmidt J, Auer M, et al. Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med. 2014;6:247ra101.
Zalutsky MR, Moseley RP, Coakham HB, et al. Pharmacokinetics and tumor localization of 131I-labeled anti-tenascin monoclonal antibody 81C6 in patients with gliomas and other intracranial malignancies. Cancer Res. 1989;49:2807–13.
Preusser M, Lim M, Hafler DA, et al. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11:504–14.
Raizer J. Issues in developing drugs for primary brain tumors: barriers and toxicities. Toxicol Pathol. 2011;39:152–7.
Dranoff G. Immunotherapy at large: balancing tumor immunity and inflammatory pathology. Nat Med. 2013;19:1100–1.
Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 2011;13:3–13.
Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16:e534–42.
Kebir S, et al. Dynamic O-(2-[18F]fluoroethyl)-l-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol. 2016;18(10):1462–4.
David A, Reardon JHS, et al. Safety and activity of nivolumab (nivo) monotherapy and nivo in combination with ipilimumab (ipi) in recurrent glioblastoma (GBM): updated results from checkmate-143. J Clin Oncol. 2016;2016:34.
Reardon DA, Omuro A, Brandes AA, et al. Randomized phase 3 study evaluating the efficacy and safety of Nivolumab vs Bevacizumab in patients with recurrent glioblastoma: checkmate 143. Neuro Oncol. 2017;19(suppl_3):iii21. doi:10.1093/neuonc/nox036.071.
Omuro A, Vlahovic G, Baehring J, et al. Nivolumab combined with radiotherapy with or without temozolomide in patients with newly diagnosed glioblastoma: results from phase 1 safety cohorts in checkmate 143. Neuro Oncol. 2016;18:vi21.
Weller M, Roth P, Preusser M, Wick W, Reardon DA, Platten M, Sampson JH. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol. 2017. doi:10.1038/nrneurol.2017.64.
Wakimoto H, Tanaka S, Curry WT, et al. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. 2014;20(11):2898–909.
Tateishi K, Wakimoto H, Iafrate AJ, et al. Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell. 2015;28(6):773–84.
Duke Comprehensive Cancer Center, Duke University. Patients with IDH1 positive recurrent grade II glioma enrolled in a safety and immunogenicity study of tumor-specific peptide vaccine. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000. https://clinicaltrials.gov/ct2/show/NCT02193347. Accessed Mar 23 2016.
National Center for Tumor Diseases, Heidelberg. Targeting IDH1R132H in WHO Grade III-IV IDH1R132H-mutated gliomas by a peptide vaccine—a phase I safety, tolerability and immunogenicity multicenter trial (NOA-16). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Franceschi, E., Minichillo, S. & Brandes, A.A. Pharmacotherapy of Glioblastoma: Established Treatments and Emerging Concepts. CNS Drugs 31, 675–684 (2017). https://doi.org/10.1007/s40263-017-0454-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-017-0454-8