Abstract
Background and Objective
Lorlatinib is a tyrosine kinase inhibitor approved for the treatment of advanced anaplastic lymphoma kinase–positive non-small cell lung cancer. This study assessed the effect of steady-state lorlatinib on the metabolic enzymes cytochrome P450 (CYP) 2B6, CYP2C9, and uridine 5′-diphospho-glucuronosyltransferase (UGT) and the P-glycoprotein (P-gp) transporter.
Methods
Thirty-two patients received a single oral dose of a probe drug on Day − 2 to determine the pharmacokinetics of the probe drug alone. Starting on Day 1, patients received 100 mg oral lorlatinib daily. On Day 15, a single oral dose of the probe drug was administered concurrently with lorlatinib. Pharmacokinetic parameters for these probe substrates were assessed.
Results
Plasma exposures of all probe substrates were reduced by lorlatinib compared with the probe alone. The greatest reduction in area under the plasma concentration–time curve from time zero to infinity (AUC∞) and maximum (peak) plasma drug concentration (Cmax) (67% and 63% decrease, respectively) was observed with the P-gp probe substrate fexofenadine. Lorlatinib coadministration also decreased the AUC∞ and Cmax of bupropion (CYP2B6 probe substrate) by 25% and 27%, tolbutamide (CYP2C9 probe substrate) by 43% and 15%, and acetaminophen (UGT probe substrate) by 45% and 28%, respectively.
Conclusions
Lorlatinib is a net moderate inducer of P-gp and a weak inducer of CYP2B6, CYP2C9, and UGT after steady state is achieved with daily dosing. Medications that are P-gp substrates with a narrow therapeutic window should be avoided in patients taking lorlatinib; no dose modifications are needed with substrates of CYP2B6, CYP2C9, or UGT.
ClinicalTrials.gov: NCT01970865.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lorlatinib was determined to be a weak inducer of cytochrome P450 (CYP) 2B6, CYP2C9, and uridine 5′-diphospho-glucuronosyltransferase and a moderate inducer of P-glycoprotein (P-gp). |
P-gp substrates with narrow therapeutic indices should be avoided. If concomitant use is unavoidable, the P-gp substrate dosage may be increased in accordance with approved product labeling. |
1 Introduction
Lorlatinib is a potent, brain-penetrant, small molecule inhibitor of anaplastic lymphoma kinase (ALK) that also potently inhibits ALK kinase domain mutations responsible for resistance to ALK inhibitor treatment in non-small cell lung cancer (NSCLC) [1]. In a phase I/II clinical study (NCT01970865), lorlatinib demonstrated a favorable safety profile, antitumor activity, and brain penetration in ALK-positive advanced NSCLC [2, 3]. In the phase III CROWN study (NCT03052608), lorlatinib demonstrated improvement in progression-free survival and a higher frequency of intracranial response versus crizotinib [4]. Based on these results, lorlatinib has been approved at a 100-mg once-daily (QD) dosage in the United States [5], the European Union [6], and other countries for first-line treatment of patients with metastatic ALK-positive NSCLC. Lorlatinib is also approved for second- and third-line settings and has utility when resistance develops to other ALK inhibitors [5, 7].
Lorlatinib is rapidly absorbed and has high bioavailability (81%) after oral administration [5]. Drug metabolism studies have shown that lorlatinib is metabolized primarily by oxidation mediated by cytochrome P450 (CYP) 3A and N-glucuronidation by uridine 5′-diphospho-glucuronosyltransferase (UGT) 1A4 with minor contributions from CYP2C8, CYP2C19, CYP3A5, and UGT1A3. M8 (PF-06895751), a benzoic acid metabolite, is the predominant, circulating, inactive metabolite in human plasma. In vitro studies have shown that lorlatinib and the M8 metabolite do not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2D6. Lorlatinib is a time-dependent inhibitor and inducer of CYP3A4/5 [5]. Following continuous multiple dosing in the clinic, lorlatinib demonstrated net autoinduction. Furthermore, lorlatinib was determined to be a net moderate CYP3A4/5 inducer after multiple dosing in a drug–drug interaction (DDI) evaluation using midazolam as the probe CYP3A4/5 substrate [8]. Since approximately 50% of prescription drugs are metabolized by CYP3A4/5 [9], the midazolam DDI assessment provides guidance for the combination of CYP3A4/5 substrates with lorlatinib. Lorlatinib has the potential of influencing the metabolism of many other medications. DDIs due to CYP3A4/5 enzyme induction may result in loss of drug efficacy or adverse drug reactions. Additionally, in a clinical evaluation with rifampin, coadministration of lorlatinib with the strong CYP3A4/5 inducer resulted in rapid elevations in transaminase values [10]. This signals the potential for hepatotoxicity. Lorlatinib in combination with strong CYP3A4/5 inducers is listed as a contraindication on the drug label [5].
The human pregnane X receptor (PXR), a nuclear receptor, is widely accepted as the principal transcriptional regulator of CYP3A4/5 induction by xenobiotics. Activation of PXR is hypothesized to induce a cascade of other drug-metabolizing enzymes [11]. Lorlatinib has been shown to activate PXR and thus has the potential to induce downstream enzymes and transporters [5]. In human cryopreserved hepatocytes, lorlatinib induced the enzymatic activity and mRNA levels of CYP2B6 and CYP3A4, indicating the likelihood of induction of these two isozymes in particular (data on file). In vitro studies conducted with human liver microsomes in the presence of bovine serum albumin indicated that UGT1A1 and UGT2B7 were inhibited at clinically relevant concentrations (data on file). Finally, lorlatinib was found to inhibit the efflux transporter P-glycoprotein (P-gp) in vitro in Madin-Darby canine kidney II-multidrug resistance protein 1 cells using the known P-gp substrate digoxin [12, 13]. Due to the simultaneous inhibition and induction properties of lorlatinib, the net effect of multiple doses of lorlatinib on substrates of CYP2B6, CYP2C9, UGT1A, and P-gp is difficult to definitively predict. Hence, a clinical evaluation of the effect of lorlatinib on these substrates was initiated, and the clinical results from these assessments are reported here. For the CYP2B6, CYP2C9, and P-gp proteins, bupropion, tolbutamide, and fexofenadine, respectively, were chosen as probe substrates in accordance with the US Food and Drug Administration (FDA) list of clinical substrates to be used for DDI studies [14]. Acetaminophen is a known UGT1A substrate and was selected as the preferred probe substrate for this substudy [15].
Complex interactions among enzymes and/or efflux transporters can only be adequately evaluated by continuous dosing of the perpetrator drug and the use of selective pharmacokinetic probes. Since multiple doses of lorlatinib cannot be administered to healthy participants, this DDI evaluation was conducted in an ongoing clinical trial of continuous dosing of lorlatinib in patients with ALK-positive or ROS proto-oncogene 1, receptor tyrosine kinase (ROS1)–positive NSCLC.
2 Methods
2.1 Trial Design and Patients
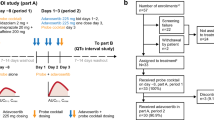
Details of the overall design of this clinical study (NCT01970865) have been previously reported [2, 3, 16]. Briefly, this ongoing, multicenter, open-label, single-arm, phase I/II trial enrolled patients with ALK-positive or ROS1-positive advanced NSCLC with or without central nervous system metastases. Patients were enrolled into six different expansion cohorts based on their ALK or ROS1 status and previous therapy with tyrosine kinase inhibitors (TKIs). The cohorts were defined as ALK treatment naive, prior crizotinib only, prior crizotinib or other TKI and one or two prior regimens of chemotherapy, two prior TKIs, three prior TKIs, and ROS1 with any prior therapy. Dose modifications were permitted to manage toxicities at the discretion of the investigator. Patients taking strong or moderate CYP3A4/5 inhibitors or strong CYP3A4/5 inducers were not eligible for inclusion [17].
The following probe substrates were used in this study: bupropion for CYP2B6, tolbutamide for CYP2C9, acetaminophen for UGT1A, and fexofenadine for P-gp.
For evaluation of the potential effect of lorlatinib on the probe substrates, six evaluable patients were required for each of the four probe substrates. Patients were administered a single dose of the probe drug on Day −2 to determine the pharmacokinetics of the probe drug in the absence of lorlatinib. Starting on Cycle 1 Day 1, lorlatinib tablets were administered orally at a dosage of 100 mg QD. On Cycle 1 Day 15, another dose of the same probe substrate was administered concurrently with daily lorlatinib dosing. The Cycle 1 Day 15 pharmacokinetic evaluation represented the disposition of the probe drug in the presence of lorlatinib.
Before participation, all patients provided written informed consent. The independent ethics committee or institutional review board at each site approved the protocol. The protocol complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws.
2.2 Pharmacokinetic Assessments
For each probe drug, 24-h serial blood samples (4 mL each at predose and 0.5, 1, 2, 3, 4, 6, 8 h, and 24 h post dose) were collected on Day − 2 and on Cycle 1 Day 15. Plasma samples were stored at approximately − 20 °C until analysis.
For bupropion as the probe substrate, samples were analyzed for bupropion and hydroxybupropion with internal standards bupropion-d9 and hydroxybupropion-d6 (stable isotopes labeled). For tolbutamide as the probe substrate, samples were analyzed for tolbutamide, hydroxytolbutamide, and 4-carboxytolbutamide with internal standards tolbutamide-d9, hydroxytolbutamide-d9, and 4-carboxytolbutamide-d9. For acetaminophen as the probe substrate, samples were analyzed for acetaminophen and acetaminophen glucuronide with internal standards acetaminophen-d3 and 4-acetaminophen β-D-glucuronide-d3 sodium salt. For fexofenadine as the probe substrate, samples were analyzed for fexofenadine and azacyclonolfexofenadine with internal standards fexofenadine-d10 and azacyclonol-d10.
Proteins were isolated from human plasma by precipitation extraction. After evaporation of the organic extracts under nitrogen, the residues were reconstituted, and the final extracts were analyzed by high-performance liquid chromatography-turbo ion spray tandem mass spectrometry using positive ionization mode.
Plasma pharmacokinetic parameters for bupropion, tolbutamide, acetaminophen, fexofenadine, and their respective metabolites hydroxybupropion [18], hydroxytolbutamide [19], carboxytolbutamide [19], acetaminophen glucuronide [20], and azacyclonolfexofenadine [21] were estimated for each patient and treatment day using noncompartmental analysis of plasma concentration–time data. Pharmacokinetic parameter values were estimated using an internally validated Pfizer software system, eNCA (version 2.2.4). Samples with plasma concentrations below the lower limit of quantitation were set to zero for parameter estimations. Actual sample collection times were used for the pharmacokinetic analysis.
2.3 Statistical Analysis
All reported pharmacokinetic parameters were summarized descriptively using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). No formal statistical testing was conducted since this DDI evaluation was not statistically powered, with approximately six evaluable patients per probe substrate empirically selected for this estimation-based assessment of the potential drug interaction(s). The estimated pharmacokinetic parameters included area under the plasma concentration–time curve (AUC) from time zero to the time of the last measurable concentration (AUClast), AUC from time zero to infinity (AUC∞), maximum (peak) plasma drug concentration (Cmax), apparent total body clearance of drug from plasma after oral administration (CL/F), time to reach the maximum (peak) plasma concentration following drug administration (Tmax), and elimination half-life (t½).
Natural log transformed AUC and Cmax for the probe substrates were analyzed using an analysis of variance (ANOVA) mixed effect model with treatment as a fixed effect and subject as a random effect. Estimates of the adjusted mean differences (test reference) and corresponding 90% confidence intervals were obtained from the model. The adjusted mean differences and 90% confidence intervals for the differences were exponentiated to provide estimates of the ratios of adjusted geometric means (test/reference) and 90% confidence intervals for the ratios.
3 Results
3.1 Patients
Thirty-two patients were enrolled in the study. Additional patients were enrolled to ensure that pharmacokinetic data were available for at least six patients in each category. Pharmacokinetic assessments were done in patients who completed the study. Overall, 53% were male, 66% were White, the average age was 54.9 ± 10.2 years, and the average weight was 69.8 ± 18.3 kg (Table 1).
3.2 Effect of Multiple-Dose Lorlatinib on the Pharmacokinetics of Bupropion (CYP2B6 Probe Substrate)
Seven patients were enrolled in the bupropion group, with six completing the study. To evaluate the effect of lorlatinib on CYP2B6 activity, bupropion, a CYP2B6 probe substrate, was administered as a single, oral dose (100 mg) alone and in combination with multiple-dose lorlatinib (100 mg QD).
Bupropion plasma concentrations in the presence of lorlatinib were lower than those observed when bupropion was administered alone, indicating net induction of CYP2B6 by continuous lorlatinib administration (Fig. 1a, Table 2).
Median plasma concentration–time profiles of a bupropion following single oral doses of bupropion alone and with once-daily lorlatinib, b tolbutamide following single oral doses of tolbutamide alone and with once-daily lorlatinib, c acetaminophen following single oral doses of acetaminophen alone and with once-daily lorlatinib, and d fexofenadine following single oral doses of fexofenadine alone and with once-daily lorlatinib
Coadministration with 100-mg QD lorlatinib decreased bupropion total plasma exposure (AUC∞) and Cmax by approximately 25% and 27%, respectively (Table 3). Strong, moderate, and weak inducers are defined as drugs that decrease the AUC of sensitive index substrates of a given metabolic pathway by ≥ 80%, ≥ 50% to < 80%, and ≥ 20% to < 50%, respectively [22]. Based on these criteria, lorlatinib is a weak inducer of the CYP2B6 enzyme after multiple dosing.
Interpatient variability for AUC∞ was 76% for bupropion alone and 43% for bupropion with lorlatinib. Interpatient variability for Cmax was 79% and 89% for bupropion alone and with lorlatinib, respectively. Peak plasma concentrations of bupropion were achieved at a median Tmax of 1 h for both bupropion alone and bupropion with lorlatinib (Table 2). The bupropion plasma t½ values could not be reliably estimated because the duration over which sampling occurred was too short relative to the projected terminal phase of the bupropion pharmacokinetic profile.
The predominant circulating metabolite of bupropion, formed via CYP2B6, is hydroxybupropion. Following administration of bupropion in combination with lorlatinib, median hydroxybupropion plasma concentrations were also lower than those observed when bupropion was administered alone (see Table 2, and Fig. S1 in the electronic supplementary material), indicating that lorlatinib likely also induces enzymes involved in the sequential metabolism of hydroxybupropion.
Of the six patients who received bupropion, a reduction in bupropion exposure was noted as expected in four patients following repeated lorlatinib dosing. However, for two patients, the reverse effect was observed, possibly due to inadvertent switching of samples between visits on Day − 2 and Cycle 1 Day 15. Hence, a separate analysis was conducted excluding the two outlier patients. To evaluate the full induction potential of lorlatinib on CYP2B6 and to avoid inappropriate data exclusions, pharmacokinetic results from both datasets are reported. The pharmacokinetic parameters excluding the two patients are shown in Tables S1 and S2 in the electronic supplementary material. The plasma concentration curves for bupropion and hydroxybupropion in this dataset are shown in supplemental Figs. S2 and S3, respectively, in the electronic supplementary material.
3.3 Effect of Multiple-Dose Lorlatinib on the Pharmacokinetics of Tolbutamide (CYP2C9 Probe Substrate)
Seven patients were enrolled in the tolbutamide group, with six completing the study. To evaluate the effect of lorlatinib on CYP2C9 activity, tolbutamide, a CYP2C9 probe substrate, was administered as a single dose alone and in combination with multiple-dose lorlatinib (100 mg QD). Following an oral dose of tolbutamide (500 mg) in the presence of steady-state lorlatinib, median tolbutamide plasma concentrations were lower than when tolbutamide was administered alone (Fig. 1b, Table 2), indicating a net induction of CYP2C9 by lorlatinib.
Peak plasma tolbutamide concentrations (Cmax) were achieved within 1–6 h post dose, with a median Tmax of approximately 2.5 h for both tolbutamide alone and with concomitant lorlatinib (Table 3). Following attainment of Cmax, tolbutamide plasma concentrations declined, with mean t½ values shorter in the presence of lorlatinib (4.0 h) compared with tolbutamide administered alone (5.9 h).
Coadministration of lorlatinib decreased tolbutamide total plasma exposure (AUC∞) and peak concentration (Cmax) by 43% and 15%, respectively. Lorlatinib is, therefore, a weak inducer of CYP2C9 after multiple dosing (Table 3).
Interpatient variability of AUC∞ was 22% for tolbutamide alone and 32% for tolbutamide with lorlatinib. Interpatient variability of Cmax was 16% and 13% for tolbutamide alone and with lorlatinib, respectively.
Hydroxytolbutamide is a tolbutamide metabolite formed via the CYP2C9 pathway. Following administration of tolbutamide, median hydroxytolbutamide plasma concentrations were similar between tolbutamide alone and tolbutamide with lorlatinib (Fig. S4, see the electronic supplementary material). Although continuous lorlatinib dosing is expected to increase the rate of formation of hydroxytolbutamide through CYP2C9 induction, the observed similar hydroxytolbutamide concentrations may indicate that lorlatinib induces the breakdown of hydroxytolbutamide to a similar extent as the lorlatinib-mediated induction of CYP2C9. Median hydroxytolbutamide Tmax was 3 h for both treatments (Table 2).
Carboxytolbutamide is another metabolite of tolbutamide formed via the subsequent metabolism of hydroxytolbutamide. Following administration of tolbutamide in combination with repeated lorlatinib dosing, median carboxytolbutamide plasma concentrations were higher than those observed when tolbutamide was administered alone (Fig. S5). One hypothesis for this observation is that lorlatinib increases the rate of formation of carboxytolbutamide via metabolic induction. Median carboxytolbutamide Tmax was approximately 4 h for both treatments (Table 2). While parent tolbutamide plasma exposure decreased in the presence of lorlatinib, carboxytolbutamide plasma exposure, as measured by geometric mean AUC from time zero to 24 h (AUC24) and Cmax, slightly increased.
3.4 Effect of Multiple-Dose Lorlatinib on the Pharmacokinetics of Acetaminophen (UGT Probe Substrate)
Nine patients were enrolled in the acetaminophen group, with seven completing the study. To evaluate the effect of lorlatinib on UGT, acetaminophen, a UGT probe substrate, was administered alone and in combination with repeated lorlatinib dosing (100 mg QD). Using the recombinant human UGT enzymes, UGT1A1, UGT1A6, and UGT1A9 have been shown to be involved in acetaminophen glucuronidation [15]. Following a single oral dose of acetaminophen (500 mg) in the presence of steady-state lorlatinib, median acetaminophen plasma concentrations were lower than those observed when acetaminophen was administered without lorlatinib (Fig. 1c).
Peak acetaminophen plasma concentrations (Cmax) were achieved within 0.5–2 h post dose, with a median Tmax of approximately 0.5 h for acetaminophen alone and with lorlatinib (Table 2). Following attainment of Cmax, plasma acetaminophen concentrations for both treatments declined in parallel, with mean t½ values of 4.4 and 3.9 h for acetaminophen alone and with lorlatinib, respectively. The interpatient variability for AUC∞ and Cmax was 52% and 33%, respectively, for acetaminophen alone and 52% and 38% following coadministration with lorlatinib.
Multiple-dose lorlatinib decreased acetaminophen AUC∞ and Cmax by 45% and 28%, respectively (Table 3). This indicates that lorlatinib is a net weak inducer of UGT.
The predominant circulating metabolite for acetaminophen via UGT is acetaminophen glucuronide. Following administration of acetaminophen in combination with lorlatinib, median acetaminophen glucuronide plasma concentrations were higher than those observed when acetaminophen was administered alone (see Table 2, and Fig. S6 in the electronic supplementary material). This observation is consistent with the lorlatinib-mediated induction of UGT, and this induction therefore increased the level of acetaminophen glucuronide.
3.5 Effect of Multiple-Dose Lorlatinib on the Pharmacokinetics of Fexofenadine (P-gp Probe Substrate)
Nine patients were enrolled in the fexofenadine group. To evaluate the effect of lorlatinib on fexofenadine, a P-gp probe substrate, fexofenadine was administered alone and in combination with repeated lorlatinib dosing (100 mg QD). Following a single oral dose of fexofenadine (60 mg) in the presence of lorlatinib, median fexofenadine plasma concentrations were lower than those observed when fexofenadine was administered alone (Fig. 1d).
Coadministration of lorlatinib decreased fexofenadine AUC∞ and Cmax by 67% and 63%, respectively (Table 3). This indicates that lorlatinib is a moderate inducer of P-gp.
Peak plasma concentrations of fexofenadine were achieved at a median Tmax of 3 h for fexofenadine alone and 2 h for fexofenadine with lorlatinib (Table 2). The t½ values were 5.7 and 6.3 h for fexofenadine alone and in combination with lorlatinib, respectively. Interpatient variability of AUC∞ was 73% for fexofenadine alone and 48% for fexofenadine with lorlatinib. Interpatient variability of Cmax was 102% and 99% for fexofenadine alone and with lorlatinib, respectively.
In addition to being a sensitive substrate for P-gp, fexofenadine also undergoes metabolism via CYP3A4/5 to form the azacyclonolfexofenadine metabolite [21]. Following fexofenadine administered in combination with repeated lorlatinib doses, median azacyclonolfexofenadine plasma concentrations were lower than with the probe substrate alone. Median plasma concentration values fell below the lower limit of quantitation (Fig. S7, see the electronic supplementary material). The median Tmax for azacyclonolfexofenadine ranged from 3 to 24 h for both treatments (Table 2).
As with the parent compound, the azacyclonolfexofenadine plasma exposures, as measured by geometric mean AUC24 and Cmax, substantially decreased in the presence of lorlatinib (Table 2). This dramatic effect suggests that while lorlatinib is likely to increase the rate of formation of azacyclonolfexofenadine via CYP3A4/5 induction, it also increases the rate of elimination of the azacyclonolfexofenadine metabolite to a greater extent.
A forest plot summarizing the effects of lorlatinib on the pharmacokinetics of the probe substrates used in this examination is provided in Fig. 2.
Forest plot illustrating the effect of daily dosing of lorlatinib on the pharmacokinetic parameters (fold change and 90% CI of the ratios of adjusted geometric means for Cmax and AUC∞) of probe substates used in this study. Adjusted geometric mean ratio refers to the estimate from the ANOVA model using a mixed effect model with treatment as a fixed effect and subject as a random effect. ANOVA analysis of variance, AUC∞/AUCinf area under the plasma concentration–time curve from time zero to infinity, CI confidence interval, Cmax maximum (peak) plasma drug concentration, CYP cytochrome P450, P-gp P-glycoprotein, UGT uridine 5′-diphospho-glucuronosyltransferase
4 Discussion
This study investigated potential DDIs of multiple-dose 100-mg QD lorlatinib (approved clinical dose) on select metabolic enzymes and drug transporter substrates. Understanding metabolic enzyme- and transporter-mediated DDIs for new therapeutics is a critical component of the drug development process, as it can provide guidance regarding concomitant use in patients in ongoing clinical trials and in clinical practice. This is especially important with novel anticancer agents since this patient population has high comedication use [22].
A common approach to evaluating DDIs with an investigational drug is the “cocktail method” [22]. In this approach, the investigational drug is co-administered with several CYP450/transporter probe substates. While efficient, this method could not be used with lorlatinib because there was a potential for lorlatinib to have complex interactions with multiple enzymes or transporters [5]. Hence, the present evaluation was conducted in four separate cohorts of study participants to assess metabolic enzymes or transporters separately. The probe substrates used for this evaluation (bupropion, tolbutamide, acetaminophen, and fexofenadine) have been validated in previous clinical studies for use as sensitive metabolic substrates for CYP2B6, CYP2C9, UGT, and P-gp, so that changes seen in their AUCs can be directly ascribed to changes in the metabolic/transporter activity. Lorlatinib is not highly protein bound (66% binding to plasma proteins), and hence, interactions based on changes in protein binding are unlikely. However, minor unknown changes in absorption could have also affected the DDI results in this study.
In addition, lorlatinib can only be administered as single doses to healthy participants due to the potential for aneugenic effects with multiple dosing to healthy participants. Lorlatinib was aneugenic in vitro in human lymphoblastoid TK6 cells and in vivo in the bone marrow of rats but was not mutagenic in an in vitro bacterial assay [5]. Therefore, the present multiple-dose lorlatinib evaluation was conducted in patients who were likely to derive clinical benefit with lorlatinib (i.e., patients with ALK-positive or ROS1-positive NSCLC).
The DDIs and probe substrates investigated in this assessment were selected on the basis of early in vitro evidence and their importance in the pharmacology of other common medications [23]. Adding further complexity, multiple oral dosing of lorlatinib is associated with autoinduction of lorlatinib metabolism [8]. CYP isozymes and drug transporters play major roles in the elimination of drugs from the body. Lorlatinib is metabolized primarily by CYP3A4 and UGT1A4, with minor contribution from CYP2C8, CYP2C19, CYP3A5, and UGT1A3 [5]. In vitro studies in human hepatocytes indicated that lorlatinib induced CYP2B6 and could lead to decreased plasma exposure of drugs that are metabolized by CYP2B6 [10]. In addition, CYP1A2, CYP2A6, CYP2C9, CYP3A4, and CYP2E1 are involved in bupropion metabolism to a lesser extent and may have contributed to the effect of lorlatinib on bupropion pharmacokinetics [24].
Consistent with the fact that CYP3A4/5 plays a major role in the metabolism of lorlatinib, clinical studies have demonstrated the effects of inducers and inhibitors of CYP3A4/5. For example, itraconazole, a strong CYP3A4/5 inhibitor, increased lorlatinib AUC∞ and Cmax by 42% and 24%, respectively. This is the basis for the current labeled recommendation to reduce the starting dosage of lorlatinib from 100 mg orally QD to 75 mg orally QD if concomitant use of strong CYP34/5A inhibitors cannot be avoided [5, 25]. Rifampin, a strong CYP3A4/5 inducer, reduced lorlatinib mean AUC∞ and Cmax by 85% and 76%, respectively, and also caused elevated liver enzyme levels in the blood [10]. Modafinil, a moderate CYP3A4/5 inducer, also decreased the plasma exposure of lorlatinib, but to a lesser extent (23% and 22% reduction in lorlatinib AUC∞ and Cmax, respectively) [26]. The use of lorlatinib with strong CYP3A4/5 inhibitors is contraindicated due to the aforementioned liver enzyme elevations with concomitant use. As for the effect of lorlatinib on other drugs, it has been demonstrated that lorlatinib can affect drugs that are CYP3A4/5 substrates. Lorlatinib 150 mg QD decreased AUC∞ by 64% and Cmax by 50% for a single oral 2-mg dose of midazolam, indicating that lorlatinib is a moderate CYP3A4/5 inducer. Hence, concomitant use of lorlatinib decreases the concentration of CYP3A4/5 substrates, which may reduce the efficacy of these substrates. This is particularly relevant in patients with cancer with frequent central nervous system metastases because many antiepileptic drugs are CYP3A4/5 substrates [27]. Concurrent use of lorlatinib with CYP3A4/5 substrates that have narrow therapeutic indices is to be avoided [5].
Evidence suggests that CYP3A4/5 and P-gp act in concert with each other in the detoxification of xenobiotics such as therapeutic medications [28]. CYP3A4/5 and P-gp expression is regulated by the nuclear receptor PXR [5, 29]. PXR activation triggers the expression of other CYP enzymes and drug transporters as well. The role of PXR agonism in DDIs has been extensively studied, and the FDA has published regulatory guidance for classification of induction potency when assessing DDI liability of novel therapeutics [28]. With the interplay of lorlatinib’s activation of PXR (leading to induction) and lorlatinib’s in vitro inhibition of various enzymes and transporters, this clinical DDI study provided an understanding on what the net effect of lorlatinib is on these metabolic enzymes and transporters.
As demonstrated in prior pharmacokinetic evaluations, lorlatinib attains steady state by 15 days of continuous daily dosing [8]. All four probe substrates given in combination with lorlatinib on Day 15 had decreased plasma exposure compared with when probe substrates were given alone on Day − 2. These results suggest that, at therapeutic concentrations, lorlatinib behaves as a net inducer of the four evaluated enzymes and transporters. As discussed, this metabolic induction is likely a consequence of PXR activation that predominated over the inhibitory effects identified in vitro. Continuous daily lorlatinib dosing had a weak inducer effect (less than 50% reduction in the AUC of the probe substrate) on CYP2B6, CYP2C9, and UGT. For P-gp, lorlatinib had a moderate inducer effect (50–80% reduction in the AUC of the probe substrate).
Polypharmacy is one of the major sources of drug-related adverse events [30]. Clinicians prescribing lorlatinib should pay particular attention to the concomitant use by their patients of P-gp substrates such as the commonly used cardiovascular drugs warfarin and digoxin [31]. It is vital that this information is shared between oncology specialists and general practitioners. In summary, the current guidance on the label is that medications that are P-gp substrates with a narrow therapeutic window should be avoided in patients taking lorlatinib as they could have decreased clinical effectiveness. If concomitant use is unavoidable, a dose increase for the P-gp substrate may be required to achieve therapeutic levels. However, in routine clinical practice, when lorlatinib is combined with substrates that have a narrow therapeutic index, dosing is usually based on titration to achieve the desired effect. For example, dosing based on therapeutic drug monitoring for digoxin or warfarin dosing based on international normalized ratio, the guided titration will lead to the choice of the appropriate dose of that substrate. No dosage modifications are necessary when combining CYP2B6, CYP2C9, and UGT substrates with lorlatinib.
References
Johnson TW, Richardson PF, Bailey S, Brooun A, Burke BJ, Collins MR, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–44.
Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel-Vinay S, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–9.
Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654–67.
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–29.
Pfizer Inc. LORBRENA® (lorlatinib): Prescribing Information. 2021 [cited 2021 August 10]. http://labeling.pfizer.com/ShowLabeling.aspx?id=11140.
European Medicines Agency. Lorviqua (lorlatinib). 2021 [cited 2021 December 1]. https://www.ema.europa.eu/en/medicines/human/EPAR/lorviqua.
Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin CC, Soo RA, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37(16):1370–9.
Chen J, O’Gorman MT, James LP, Klamerus KJ, Mugundu G, Pithavala YK. Pharmacokinetics of lorlatinib after single and multiple dosing in patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer: results from a global phase I/II study. Clin Pharmacokinet. 2021;60(10):1313–24.
Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov. 2002;1(4):259–66.
Chen J, Xu H, Pawlak S, James LP, Peltz G, Lee K, et al. The effect of rifampin on the pharmacokinetics and safety of lorlatinib: results of a phase one, open-label, crossover study in healthy participants. Adv Ther. 2020;37(2):745–58.
Wang YM, Ong SS, Chai SC, Chen T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8(7):803–17.
Li W, Sparidans RW, Wang Y, Lebre MC, Wagenaar E, Beijnen JH, et al. P-glycoprotein (MDR1/ABCB1) restricts brain accumulation and cytochrome P450–3A (CYP3A) limits oral availability of the novel ALK/ROS1 inhibitor lorlatinib. Int J Cancer. 2018;143(8):2029–38.
Verschraagen M, Koks CH, Schellens JH, Beijnen JH. P-glycoprotein system as a determinant of drug interactions: the case of digoxin-verapamil. Pharmacol Res. 1999;40(4):301–6.
US Food and Drug Administration (FDA). Drug Development and Drug Interactions | Table of Substrates, Inhibitors and Inducers. 2023 [cited 2023 April 26]. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers#table2-1.
Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther. 2005;106(1):97–132.
Shaw AT, Solomon BJ, Chiari R, Riely GJ, Besse B, Soo RA, et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019;20(12):1691–701.
Chen J, O’Gorman MT, James LP, Klamerus KJ, Mugundu G, Pithavala YK. Pharmacokinetics of lorlatinib after single and multiple dosing in patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer: results from a global phase I/II study. Clin Pharmacokinet. 2021;60(10):1313–24.
Wellbutrin. Package insert. GlaxoSmithKline. 2019.
Miners JO, Birkett DJ. Use of tolbutamide as a substrate probe for human hepatic cytochrome P450 2C9. Methods Enzymol. 1996;272:139–45.
Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S-S298.
Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69(3):114–21.
US Food and Drug Administration (FDA). Guidance for Industry. Clinical Drug Interaction Studies—Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) January 2020 Clinical Pharmacology.
Ford SL, Sutton K, Lou Y, Zhang Z, Tenorio A, Trezza C, et al. Effect of rifampin on the single-dose pharmacokinetics of oral cabotegravir in healthy subjects. Antimicrob Agents Chemother. 2017;61(10):1128.
Jefferson JW, Pradko JF, Muir KT. Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations. Clin Ther. 2005;27(11):1685–95.
Patel M, Chen J, McGrory S, O’Gorman M, Nepal S, Ginman K, et al. The effect of itraconazole on the pharmacokinetics of lorlatinib: results of a phase I, open-label, crossover study in healthy participants. Invest New Drugs. 2020;38(1):131–9.
Li J, Pithavala YK, Gong J, LaBadie RR, Mfopou JK, Chen J. The effect of modafinil on the safety and pharmacokinetics of lorlatinib: a phase I study in healthy participants. Clin Pharmacokinet. 2021;60(10):1303–12.
Bénit CP, Vecht CJ. Seizures and cancer: drug interactions of anticonvulsants with chemotherapeutic agents, tyrosine kinase inhibitors and glucocorticoids. Neuro-Oncology Pract. 2015;3(4):245–60.
Lutz JD, Kirby BJ, Wang L, Song Q, Ling J, Massetto B, et al. Cytochrome P450 3A induction predicts P-glycoprotein induction; part 1: establishing induction relationships using ascending dose rifampin. Clin Pharmacol Ther. 2018;104(6):1182–90.
Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23(5):687–702.
Matsuyama T, Tachi T, Katsuno H, Sugioka M, Aoyama S, Osawa T, et al. Effects of polypharmacy on the prevalence of adverse drug events resulting in outpatient visits and hospitalization. Pharmazie. 2021;76(6):279–86.
Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol. 2013;61(25):2495–502.
Acknowledgements
This study was sponsored by Pfizer. Editorial and medical writing support was provided by Mark McCollum, Ph.D., of Clinical Thinking, Inc., and was funded by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
JC, HT, JSC, KL, MO, CT, and YKP analyzed the data. AB, D-WK, HM, JB, RC, S-HIO, BJS, RAS, EF, and AS collected the data. All authors contributed to the interpretation of the data and the development, writing, and approval of the manuscript.
Funding
This study was sponsored by Pfizer.
Conflict of interest
JC was employed by Pfizer at the time of the work, owns stocks in Pfizer, and is currently employed by Roche/Genentech. AB reports advisory board fees from AstraZeneca, Bristol Myers Squibb, Pfizer, and Roche; speaker fees from Eli Lilly, Pfizer, and Roche. D-WK reports administrative support and grants from Amgen, AstraZeneca, Boehringer Ingelheim, Bridge BioTherapeutics, Chong Keun Dang, Daiichi Sankyo, GlaxoSmithKline, Janssen, Merck, Merus, MSD, Novartis, Pfizer, Roche, Takeda, and Yuhan; grants from Alpha Biopharma, Hanmi, InnoN, Mirati Therapeutics, ONO Pharmaceutical, Turning Point Therapeutics, and Xcovery. HM reports advisory board fees from AstraZeneca, Genentech, and Zentalis; received a grant from U CAN-CER VIVE Foundation; and received institutional research funding from Pfizer. JB reports consulting fees or honoraria from BeiGene, Blueprint Medicines, Eli Lilly, Janssen, Merck, Mirati, Pfizer, and Turning Point Therapeutics. RC declares no conflicts of interest. S-HIO reports consulting fees or honorarium from AnHeart Therapeutics, BeiGene, Daiichi Sankyo, Eli Lilly, Johnson and Johnson/Janssen, and Pfizer; speaker fees from DAVA Oncology, Johnson and Johnson/Janssen, and Pfizer; advisory board fees from Elevation Oncology; stocks in Elevation Oncology and Turning Point Therapeutics. BJS reports advisory board fees or honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Merck, Pfizer, Roche/Genentech; and received fees from BeiGene, Janssen, Novartis, and Takeda. RAS reports advisory board fees or honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, EMD Serono, J INTS BIO, Janssen, Merck, Microquin, Novartis, Pfizer, Puma Biotechnology, Roche, Taiho, Takeda, ThermoFisher, and Yuhan; and research grants from AstraZeneca and Boehringer Ingelheim. EF reports consulting fees or honoraria from Amgen, AstraZeneca, BerGenBio, Bristol Myers Squibb, Daichi Sankyo, Eli Lilly, F. Hoffman-La Roche, GlaxoSmithKline, Janssen, Merck Serono, MSD, Novartis, Peptomyc, Pfizer, Sanofi, and Takeda; speaker fees from Amgen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, F. Hoffman-La Roche, Janssen, Medical Trends, MedScape, MSD, Novartis, PeerVoice, Pfizer, Sanofi, Takeda, and Touch Oncology; and is an independent member of the board at Grifols. AS is employed by and owns stock in Novartis. HT, JSC, KL, MO, CT, and YKP are employed by and own stocks in Pfizer.
Data Sharing Statement
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics approval
The independent ethics committee or institutional review board at each site approved the protocol. The protocol complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws.
Consent to participate
Before participation, all patients provided written informed consent.
Consent for publication
All authors had the final responsibility for the decision to submit the publication.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, J., Bearz, A., Kim, DW. et al. Evaluation of the Effect of Lorlatinib on CYP2B6, CYP2C9, UGT, and P-Glycoprotein Substrates in Patients with Advanced Non-Small Cell Lung Cancer. Clin Pharmacokinet 63, 171–182 (2024). https://doi.org/10.1007/s40262-023-01309-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01309-4