Abstract
Background and Objective
As a result of changes in physiology during pregnancy, the pharmacokinetics (PK) of drugs can be altered. It is unclear whether under- or overexposure occurs in pregnant cancer patients and thus also whether adjustments in dosing regimens are required. Given the severity of the malignant disease and the potentially high impact on both the mother and child, there is a high unmet medical need for adequate and tolerable treatment of this patient population. We aimed to develop and evaluate a semi-physiological enriched model that incorporates physiological changes during pregnancy into available population PK models developed from non-pregnant patient data.
Methods
Gestational changes in plasma protein levels, renal function, hepatic function, plasma volume, extracellular water and total body water were implemented in existing empirical PK models for docetaxel, paclitaxel, epirubicin and doxorubicin. These models were used to predict PK profiles for pregnant patients, which were compared with observed data obtained from pregnant patients.
Results
The observed PK profiles were well described by the model. For docetaxel, paclitaxel and doxorubicin, an overprediction of the lower concentrations was observed, most likely as a result of a lack of data on the gestational changes in metabolizing enzymes. For paclitaxel, epirubicin and doxorubicin, the semi-physiological enriched model performed better in predicting PK in pregnant patients compared with a model that was not adjusted for pregnancy-induced changes.
Conclusion
By incorporating gestational changes into existing population pharmacokinetic models, it is possible to adequately predict plasma concentrations of drugs in pregnant patients which may inform dose adjustments in this population.
Similar content being viewed by others
As a result of short-term fetal and maternal safety data, chemotherapy is increasingly used to treat pregnant patients with cancer. As pregnant patients are typically excluded from clinical trials, there is a high unmet medical need for adequate and tolerable dosing regimens in this patient population. By incorporating gestational changes into existing population pharmacokinetic models, it is possible to adequately predict plasma concentrations of drugs in pregnant patients which may inform dose adjustments in this population. |
1 Introduction
Cancer is manifested in one out of 1000 pregnancies. Recently, it has been shown that oncological treatment during pregnancy, under strict guidelines and precautions, is safe and thus recommended [1]. An increased use of chemotherapy during pregnancy has been associated with an increased number of live births. Nevertheless, as pregnant patients are typically excluded from clinical trials and post-marketing studies in pregnant patients are rarely performed, information on the appropriateness of dosing strategies during pregnancy is missing [2].
During pregnancy, pharmacokinetics (PK) of drugs can be altered as a result of various changes in several physiological processes. Over the past years, efforts have been made to quantify these physiological alterations during pregnancy [3, 4]. Firstly, a gradual decrease in plasma levels for both albumin and α1-acid-glycoprotein over the course of pregnancy has been reported. Furthermore, it has been shown that the glomerular filtration rate (GFR) increases until the third trimester but then slightly decreases again during late pregnancy. A 1.5-fold increase in total body water during pregnancy has been shown, as well as an increase in body fat, which may result in alterations in distribution volumes. Nevertheless, for other parameters, such as drug metabolizing enzymes, limited and, on occasion, conflicting data exist. These changes may result in increased or decreased drug concentrations compared with non-pregnant women, and this may change over the course of pregnancy [3].
Given the small therapeutic window of most cytotoxic drugs, small changes in concentrations of cytotoxic agents may influence the therapeutic effect. A major concern is that lower drug plasma concentrations in pregnant women might result in a negative effect on survival. However, results from small cohorts of pregnant patients with breast cancer, cervical cancer and Hodgkin lymphoma have shown that cancer prognoses are similar to those of non-pregnant patients [5,6,7].
An empirical PK analysis of four cytotoxic agents obtained from pregnant cancer patients indeed showed alterations in PK parameter estimates compared with non-pregnant patients [8]. Given the complexity of the physiological changes during pregnancy, the magnitude and relevance of these alterations on the PK of anticancer drugs is not straightforward. Ideally, PK studies should be performed to quantify these changes. However, clinical studies in pregnant women are difficult to perform as a result of the low incidence of malignancies in women of child-bearing potential, and the many variables that should be taken into account [gestational age, cancer (sub)type, treatment regimen, co-medication] [9]. Nevertheless, there is a high unmet medical need for adequate and tolerable treatment of this neglected patient population.
Whole-body physiologically based PK models can be used to predict pregnancy-induced changes in PK [10]. However, for most cytotoxic agents, extensive knowledge on the PK of the drug in non-pregnant patients is available mostly in the form of empirical population PK models. With this work, we aimed to develop a methodology in which the advantages of physiologically based PK models are combined with relevant existing knowledge of the PK in non-pregnant patients, enabling the prediction of individual PK profiles of a range of cytotoxic drugs in pregnant patients. To this end, we implemented a semi-physiological enriched pregnancy model including changes over the gestational time that allows the prediction of the PK of cytotoxic drugs in pregnant women using only available empirical compartmental models based on non-pregnant patient PK data. To evaluate the physiologically enriched model, we used PK data from pregnant women who were treated with either doxorubicin, epirubicin, docetaxel or paclitaxel, collected internationally over the course of 10 years.
2 Methods
2.1 Development of a Semi-physiological Enriched Model
Physiological changes over time during the course of pregnancy have extensively been described in the literature [3, 4]. To describe the typical change in PK parameters during pregnancy, a selection of relevant empirical equations for physiological changes from Abduljalil et al. were implemented in our semi-physiological predictions [3]. This work includes all relevant physiological parameters that might have an influence on the PK of either doxorubicin, epirubicin, docetaxel or paclitaxel.
2.1.1 Plasma Proteins
Plasma protein levels, such as albumin and alpha1-acid glycoprotein (AAG), decrease during pregnancy, which may affect the unbound concentration of drugs that are highly protein bound. The unbound plasma concentration affects the pharmacological effect of the drug and it is mostly the unbound drug fraction that is renally or hepatically eliminated [3]. The following empirical equations describe the serum albumin concentration (Calb) (Eq. 1) and serum AAG concentration (CAAG) (Eq. 2) as a function of estimated gestational age (EGA):
To determine the change in unbound drug concentration (fu) over time during pregnancy, the dissociation constant (kD) was calculated by Eq. (3) and assumed to remain constant during the complete pregnancy period. Subsequently, Eq. (4) was used to determine the expected change in free unbound drug fraction during pregnancy, for which it was assumed that the free drug concentrations are much lower than kD and that the maximal binding capacity per protein molecule is 1. This would imply linear protein binding and thus an fu independent of the free drug concentration.
where Cprotein is either Calb or CAAG as derived from Eqs. (1) or (2). Previously established drug-specific non-pregnant (i.e. EGA = 0 weeks) percentage of protein binding was used for fu (EGA = 0) (Table 1).
2.1.2 Clearance
Clearance can be subdivided into the two main routes, renal (CLR) and hepatic (CLH) clearance as follows:
Many changes occur in the urinary and hepatic system during pregnancy, including an increase in GFR, enhanced creatinine clearance (CLCR) and hepatic blood flow (QH,blood). Also, variable changes in activities of drug-metabolizing enzymes have been reported. Since all four studied drugs are at least partially eliminated by glomerular filtration and only the unbound drug fraction is eliminated, the change in renal clearance during pregnancy was defined by Eq. (6), in which GFR was described by Eq. (7) and CLR(EGA = 0) is the reported renal clearance in non-pregnant patients.
Limited and contradictory data are reported on the change in QH,blood during pregnancy, we therefore assumed that the hepatic blood flow remained unchanged over pregnancy and was thus fixed to a typical non-pregnant value of 109 L/h [11]. Both changes in unbound fraction and changes in QH,plasma which change during pregnancy can influence CLH. A decrease in haematocrit (HCT) is observed during pregnancy, which is described by Eq. (8):
Subsequently, the hepatic plasma flow (QH,plasma) can be described with Eq. (9):
To describe the relationship between CLH, the changing fraction unbound, the hepatic plasma flow (QH,plasma) and intrinsic clearance (CLint), the following well-stirred liver model equation was used:
Equation (10) was rearranged to determine the CLint in non-pregnant patients, using previously estimated drug-specific values of CLH (Eq. 11).
To account for the changes in activities of enzymatic pathways during pregnancy, CLint was corrected for the change in enzyme activity (E):
where E(EGA) represents the enzyme activities of relevant enzymes during pregnancy, and EGA = 0 is the enzyme activity in the non-pregnant state (i.e. 100%). The change in drug-metabolizing enzyme activity of CYP3A4 was described as follows:
Although the activity of CYP2C8 and UGT2B7 might be increased during pregnancy, little is known about the magnitude of this increase [3]. We therefore assumed that the activity of these enzymes remained unchanged over pregnancy.
2.1.3 Volume of Distribution
We used the relationship between distribution volume (Vd), plasma volume (Vplasma) and a drug-specific metric for total body fluids (VE) minus Vplasma, as previously proposed by Gibaldi and McNamara [12]:
where fu is the fraction of unbound drug in plasma as described in Eq. (4), ft is the unbound drug fraction in tissue and fu/ft represents the tissue partition coefficient. fu/ft was assumed to remain constant during pregnancy since it is expected that the change in plasma proteins affects fu and ft to a similar extent. An increase in Vplasma is observed during pregnancy, which is described by Eq. (15):
Apparent volumes of distribution were expected to change during pregnancy. Reported non-pregnant volumes of distribution were scaled to the pregnant state using Eqs. (14–16). Vd is the volume of distribution of interest and VE is represented by either total body water (TBW) or extracellular water (ECW), depending on the extent of the volumes of distribution:
2.2 Prediction
Pharmacokinetic data for doxorubicin, epirubicin, docetaxel and paclitaxel were available from a prospective multinational and multicentre clinical study investigating the effects of the administration of chemotherapy during pregnancy. Pharmacokinetic and clinical results from this trial were reported previously [1, 8]. Concentration–time curves from 26, 16, 9 and 19 pregnant patients were available for doxorubicin, epirubicin, docetaxel and paclitaxel, respectively.
We searched the literature to obtain non-linear mixed-effects population PK models that adequately described the PK of the four cytotoxic drugs in non-pregnant patients [13,14,15,16]. These models were extended with the above described semi-physiological gestational changes to provide PK predictions for pregnant individuals. Covariates were excluded from the predictions to predict the typical change in PK parameters during pregnancy. Drug-specific PK characteristics that were taken into account, such as protein binding and routes of metabolism, are presented in Table 1. With these PK parameters, typical concentration–time profiles were predicted for EGAs that matched the observed dataset. To provide a valid comparison to the observed concentration–time profiles, the dosing regimens from the clinical data were used. Since dosing of doxorubicin, epirubicin, docetaxel and paclitaxel is based on a patients’ body surface area (BSA), the median BSA found in the studied patients was used (Table 2).
2.3 Evaluation
The observed concentration–time profiles available from the clinical study were used to visually evaluate the performance of the semi-physiological enriched models. Secondly, individual model fits and predictions were obtained for the observed pharmacokinetic data from pregnant women based on individual Bayesian estimates which were obtained by using non-linear mixed-effects modelling, more specifically by using the MAXEVAL = 0 and POSTHOC options in NONMEM® [17]. The fit of the semi-physiological model during pregnancy was compared to the fit of the model parameters for the non-pregnant state (EGA = 0). Subsequently, changes in the objective function value (ΔOFV, corresponding to minus twice the log-likelihood) were evaluated to compare and assess both models.
2.4 Software
Predictions were performed using a differential equation system in R (version 4.2.1) together with the R package deSolve [18]. NONMEM® v.7.5 (ICON Development Solutions, Ellicott City, MD, USA) was used to evaluate the developed model [17].
3 Results
3.1 Docetaxel
The PK of intravenously administered docetaxel has adequately been described by a linear three-compartment model with linear elimination [13]. Docetaxel is mainly metabolized by CYP3A4 and thereafter eliminated as inactive metabolites in the faeces. Hence, the change in CYP3A4 enzyme activity during pregnancy was incorporated in the predictions. As docetaxel is mainly bound to AAG in the plasma, Eq. (2) was incorporated to account for protein binding. Tables 3 and 4 show the typical change in the docetaxel primary and secondary PK parameters, respectively, during pregnancy. The largest increase for the primary PK parameters was observed for V3 in the third trimester. However, the largest effect in in secondary parameter was observed for AUC0–48h in the second and third trimester, suggesting that change in CL is more clinically relevant than in V3.
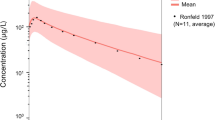
Typical concentration–time curves were predicted for the median gestational age (GA), which was 32 weeks for the patients treated with docetaxel. Although trough concentrations were slightly overpredicted, the typical observed pregnant concentrations were well described by the semi-physiological enriched model (Fig. 1). Additionally, the predictions clearly demonstrated that the use of non-pregnant parameter estimates resulted in an overprediction of the observed concentrations (Fig. 1). Comparison of the model fit for the individual predictions based on the semi-physiological pregnant parameter estimates versus non-pregnant parameter estimates resulted in an increase in OFV of 70.1 points.
3.2 Paclitaxel
For paclitaxel, a population PK model that consists of a three-compartment model with saturable distribution to the first peripheral compartment and saturable clearance for the central compartment has been published [14]. Paclitaxel is mainly bound to albumin in plasma, metabolized by both CYP3A4 and CYP2C8, and partly eliminated by the faeces. Hence, the change in albumin plasma levels and CYP3A4 enzyme activity were used. Data on the gestational change in CYP2C8 activity are still lacking and could therefore not be included. To account for the change in clearance of paclitaxel during pregnancy, the maximal elimination rate (VMEL) was scaled according to Eqs. (10–13). Typical parameter changes were similar to the changes that were observed for docetaxel. All volumes of distribution increased during pregnancy, with a maximum increase of approximately 60% for V3. Secondary PK parameters were mainly affected in both the second and third trimester, with a predicted decrease of approximately 10%. Concentration–time curves were predicted for the median GA of 31 weeks that was observed in the evaluation dataset (Fig. 1). The semi-physiological predictions adequately described the observed paclitaxel concentrations. A minor overprediction of the observed concentrations between pre-dose and 5 h after administration was observed. Notwithstanding, an overprediction of the observations was evident when non-pregnant estimates were used, especially in the elimination phase of the concentration–time curve. The model fit for the individual predictions based on the semi-physiological pregnant parameter estimates showed decrease of the OFV of 279.3 compared with non-pregnant parameter estimates.
3.3 Doxorubicin
The PK of doxorubicin has previously been described by a two-compartment model with linear clearance and extensive distribution (Table 1) [15]. Doxorubicin is metabolized by CYP3A4 into both active and inactive metabolites, which are mainly excreted by the faeces. Small initial increases ranging from 3.8 to 10.4% were observed for CL and V1. The largest increase was observed for V2, which increased by 46.3% in the third trimester. However, just as for docetaxel the largest clinically relevant decrease in secondary PK parameters was the AUC0–48h in the second and third trimester.
Typical concentration–time curves were predicted for the median GA, which was 29 weeks for the patients treated with doxorubicin. Although doxorubicin concentrations were still slightly overpredicted in the terminal elimination phase, this overprediction was smaller than for the non-pregnant model-based predictions and concentrations were well described in the initial part of the elimination phase until about 5 h after administration.
Figure 1 clearly demonstrates that predictions using model parameters based on the non-pregnant state resulted in an overprediction of the observed doxorubicin concentrations. In addition, the model fit for the individual predictions based on the semi-physiological pregnant parameter estimates showed a decrease of the OFV of 74.0 compared with non-pregnant parameter estimates.
3.4 Epirubicin
For epirubicin, a three-compartment model with linear clearance and extensive tissue distribution has been published [16]. Epirubicin is metabolized by CYP3A4, glucuronidated and thereafter excreted by the faeces. For epirubicin, typical parameter increases in CL and V1 were comparable with the observed increases in doxorubicin CL and V1. However, V2 and V3 showed a larger increase then was observed for doxorubicin V2. Secondary PK parameters were hardly affected, with the largest decrease in AUC0–48h in the third trimester of 6.08%. Concentration–time curves were predicted for the median observed GA of 27 weeks. The semi-physiological enriched model adequately predicted the epirubicin concentration-time curves that were observed during pregnancy. Additionally, the predictions using non-pregnant estimates showed an overprediction of the observed concentration–time curve. Comparison of the model fit for the individual predictions based on the non-pregnant versus the semi-physiological pregnant parameter estimates showed a significantly improved fit for the latter, with a decrease in OFV of 21.2 points observed for epirubicin.
4 Discussion
With this work, we have demonstrated the feasibility and relevance of a semi-physiological prediction approach in which prior knowledge of both the human population PK of a cytotoxic drug and physiological changes during pregnancy are combined to predict changes in PK in pregnant patients. To this end, we used the physiological alterations during pregnancy that have been described in a quantitative manner by Abduljalil et al. [3].
The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) provided guidance on the use of medicinal products during pregnancy [2, 19, 20]. In these guidelines, it is stated that post-marketing studies should be performed and exposure registries should be established for drugs that are used in women of child-bearing age. With these studies, data on the outcomes of pregnancies exposed to these drugs are collected. The EMA additionally advises to use a population PK approach to identify potential gestational effects and hence simulate doses that achieve PK exposure in pregnant patients similar to non-pregnant patients.
An alternative widely used approach is the development of a full physiologically based pharmacokinetic model. These models are constructed from known anatomical and physiological characteristics (system-related parameters) combined with physicochemical and clinical pharmacological drug characteristics (drug-related parameters). With these models, extrapolation to unstudied conditions can be made e.g. for drug–drug interactions, use of drugs in children or in pregnant patients. However, when drugs are introduced for the treatment of pregnant patients, clinical information on the drug’s disposition and elimination in non-pregnant patients is generally already available in the form of empirical population PK models and can be of great value to inform the extrapolation to pregnant patients. The here-presented semi-physiological enriched model implements a hybrid methodology that integrates actual patient data with the quantitative and longitudinal physiological changes during pregnancy. This latter aspect is comparable to the use of system-related parameters in physiologically based pharmacokinetic models. This provides a first prediction of the PK profile in pregnant women based on their GA, taking into account established non-pregnant empirical PK models. This is particularly relevant for cytotoxic agents as their application in pregnant cancer patients is rare and difficult due to their mutagenic and teratogenic potential, but nevertheless optimal treatment is sometimes required for maternal survival [1].
For docetaxel, the semi-physiological approach did not perform better than the non-pregnant PK model parameters and only modest changes in typical parameters were observed. This shows that pregnancy has a limited effect on the PK of docetaxel and predictions using the parameters based on non-pregnant data perform well for the pregnant population. In an empirical population PK analysis, we found small changes in docetaxel PK during pregnancy as well. For the paclitaxel 80 mg/m2 dose regimen, the predictions showed an overprediction of the concentrations. This could be explained by the very small sample size and wide gestational age range in the pregnancy dataset that was available for evaluation. In addition to CYP3A4, paclitaxel is metabolized by CYP2C8, for which no relationship with gestational age is available due to a lack of data on the dynamics of this enzyme. An underestimation of the CYP2C8 activity during pregnancy could in theory be a potential cause for the observed overprediction. Furthermore, the PK of taxanes is characterized by large inter-individual variability which complicates the identifiability of an effect of gestation in a small sample size. The predicted epirubicin concentrations were in good agreement with the observations, although some overprediction of the trough concentrations was observed. Epirubicin is metabolized by both CYP3A4 and UGT2B7. For UGT2B7 increased activity during pregnancy has been reported but this has not been quantified [3]. The assumption of unchanged UGT2B7 activity during pregnancy could fairly result in an underprediction of the clearance of epirubicin. For doxorubicin, we used a previously described two-compartment model that was developed using sparsely collected PK data. Predictions with this non-pregnant model for the pregnant population resulted in an overprediction of the observed concentrations in the terminal elimination phase. The observed, rich-sampled data indicate that a three-compartment model might be better suitable to describe doxorubicin PK. Nevertheless, the semi-physiological approach performed significantly better than the model-based predictions based on non-pregnant parameter estimates. Overall, our semi-physiological enriched model provided a reasonable prediction of the PK in women at any stage of gestation for various cytotoxic drugs. In line with previous findings, lower drug concentrations were predicted during pregnancy compared with the non-pregnant state, and therefore, clinical implications described previously also apply here [21]. Moreover, the semi-physiological model resulted in a significant better fit of the PK data from pregnant patients than the literature-based models for paclitaxel, epirubicin and doxorubicin.
This semi-physiological enriched model is depending on assumptions and simplifications which might all pose limitations. Firstly, we only accounted for changes in maternal PK and did not include changes related to the foetal compartment. It has been shown that the placenta is a good protector for taxanes and anthracyclines. In animal studies, foetal paclitaxel plasma concentrations were approximately 1% of the maternal concentrations while foetal plasma docetaxel levels were not detectable. In addition, placental passage was 4% for epirubicin and 8% for doxorubicin [9]. This suggests limited distribution to the foetal compartment of the here-investigated chemotherapeutics. However, the semi-physiological based model could easily be extended with a foetal compartment for drugs that cross the placenta. Consequently, foetal exposure can be predicted, based on the predicted maternal PK. Secondly, we did not incorporate changes in transporters and their possible implications on clearance. Transporters that could be explored in further updates of the model are P-gp and OATP1B3, of which docetaxel and paclitaxel are known substrates. Also, to extend our model to other drugs with other key disposition determinants than described in our model (such as transporters), first a relation between gestational age and key disposition determinants in the form of an empirical equation has to be available before it can be implemented. Thirdly, we assumed unchanged partition coefficients that account for the distribution between body compartments. As the proportion of body fat shows a typical increase in addition to alterations in body fluids, this assumption might lead to an underprediction of the pregnant volumes of distribution. The hepatic blood flow was also assumed to remain constant during pregnancy. Contradictory results have been published regarding the change in hepatic blood flow during pregnancy. In these studies, different measurement methods were applied resulting in highly variable and inconsistent results. In addition, the relationship between the gestational induced change in other cardiovascular parameters such as cardiac output and the hepatic blood flow remains unclear [3]. Hence, the change in hepatic clearance during pregnancy was driven by the change in metabolizing enzyme activity. A maternal increase in CYP3A4 activity has been shown in several studies and was, therefore, included in our semi-physiological enriched model. The activity of UGT2B7 has been suggested to increase over pregnancy as well, but the magnitude of this increase is still not quantitatively described [3]. Changes in CYP2C8 could not be incorporated because no relationships with gestation have been established. Consequently, non-pregnant metabolizing activity was assumed for these enzymes over pregnancy. Also, it should be noted that the developed semi-physiological model relies on the validity and accuracy of the estimates from the non-pregnant PK studies. In addition, we assumed similar variability in PK parameters between pregnant versus non-pregnant patients and used the typical parameter estimates for predictions. High variability and bias in these estimates could potentially result in inadequate predictions of the pregnant PK. It should be noted, however, that when relevant changes in these parameters are described, they can be implemented in this model similarly to the other implemented changes. In this respect, the semi-physiological enriched model developed here incorporates similar processes as full physiologically based pharmacokinetic models.
5 Conclusions
The semi-physiological enriched model provided an adequate prediction of the PK for four cytotoxic agents of two distinct drug classes in women over varying stages of gestation. It can be concluded that this proof of principle for a semi-physiological enriched model is applicable to the four cytotoxic drugs in our manuscript and can be extended to drugs with different pharmacological characteristics by the addition of relevant metabolic properties. This method may therefore be used for extrapolation purposes to adjust dosing regimens in pregnant women for drugs for which PK data from pregnant women are unavailable.
References
de Haan J, Verheecke M, Van Calsteren K, Van CB, Shmakov RG, Gziri MM, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19:337–46.
European Medicines Agency (EMA). Guideline on the exposure to medicinal products during pregnancy: need for post-authorization data (2006).
Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51:365–96.
Dallmann A, Ince I, Meyer M, Willmann S, Eissing T, Hempel G. Gestation-specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet. 2017;56:1303–30.
Amant F, Vandenbroucke T, Verheecke M, Fumagalli M, Halaska MJ, Boere I, et al. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med. 2015;373:1824–34.
Halaska MJ, Uzan C, Han SN, Fruscio R, Dahl Steffensen K, Van Calster B, et al. Characteristics of patients with cervical cancer during pregnancy: a multicenter matched cohort study. An initiative from the International Network on Cancer, Infertility and Pregnancy. Int J Gynecol Cancer. 2019. (Online ahead of print).
Maggen C, Dierickx D, Lugtenburg P, Laenen A, Cardonick E, Smakov R, et al. Obstetric and maternal outcomes in patients diagnosed with Hodgkin lymphoma during pregnancy: a multicentre, retrospective, cohort study. Lancet Haematol. 2019;6:e551–61.
Janssen JM, Van Calsteren K, Dorlo TPC, Halaska MJ, Fruscio R, Ottevanger P, et al. Population pharmacokinetics of docetaxel, paclitaxel, doxorubicin and epirubicin in pregnant women with cancer: a study from the International Network of Cancer, Infertility and Pregnancy (INCIP). Clin Pharmacokinet. 2021. (Online ahead of print).
Vandenbroucke T, Verheecke M, Fumagalli M, Lok C, Amant F. Effects of cancer treatment during pregnancy on fetal and child development. Lancet Child Adolesc Health. 2017;1:302–10.
Dallmann A, Mian P, den Anker J, Van AK. Clinical pharmacokinetic studies in pregnant women and the relevance of pharmacometric tools. Curr Pharm Des. 2019;25:483–95.
Nakai A, Sekiya I, Oya A, Koshino T, Araki T. Assessment of the hepatic arterial and portal venous blood flows during pregnancy with Doppler ultrasonography. Arch Gynecol Obstet. 2002;266:25–9.
Gibaldi M, McNamara PJ. Apparent volumes of distribution and drug binding to plasma proteins and tissues. Eur J Clin Pharmacol. 1989;13:373–80.
Koolen SLW, Oostendorp RL, Beijnen JH, Schellens JHM, Huitema ADR. Population pharmacokinetics of intravenously and orally administered docetaxel with or without co-administration of ritonavir in patients with advanced cancer. Br J Clin Pharmacol. 2010;69:465–74.
Crombag M-RBS, de VriesSchultink AHM, Koolen SLW, Wijngaard S, Joerger M, Schellens JHM, et al. Impact of older age on the exposure of paclitaxel: a population pharmacokinetic study. Pharm Res. 2019;36:33.
Joerger M, Huitema ADR, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, et al. Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet. 2007;46:1051–68.
Sandstrom M, Lindman H, Nygren P, Johansson M, Bergh J, Karlsson M. Population analysis of the pharmacokinetics and the haematological toxicity of the fluorouracil-epirubicin-cyclophosphamide regimen in breast cancer patients. Cancer Chemother Pharmacol. 2006;58:143–56.
Bauer RJ. Nonmem users guide introduction to Nonmem 7.3.0. ICON Development Solutions, 2014.
RC Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009.
FDA Guidance for Industry. Pharmaockinetics in pregnancy—study design, data analysis, and impact on dosing and labeling. FDA Guidance (2004).
FDA Guidance for Industry. establishing pregnancy exposure registries. FDA Guidance (2002).
van Hasselt JGC, Van Calsteren K, Heyns L, Han S, MhallemGziri M, Schellens JHM, et al. Optimizing anticancer drug treatment in pregnant cancer patients: pharmacokinetic analysis of gestation-induced changes for doxorubicin, epirubicin, docetaxel and paclitaxel. Ann Oncol. 2014;25:2059–65.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
JMJ, JGCH, DD, FA, KC, JHB, ADRH and TPCD wrote the manuscript; TPCD and ADRH designed the research; JMJ and DD performed the research; JMJ analysed the data.
Funding
Open access funding provided by Uppsala University. Funding for the INCIP registry, sample bioanalysis and sample logistics was provided by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 647047), the Research Foundation-Flanders and the Belgian Cancer Plan, Ministry of Health, Belgium. Kristel Van Calsteren received a clinical research fund from the University Hospitals Leuven. Thomas P.C. Dorlo was personally supported by a Dutch Research Council (NWO)/ZonMw Veni grant.
Conflict of interest
Frédéric C.H. Amant is a senior clinical investigator for the Research Fund-Flanders. All other authors declared no competing interests for this work.
Ethics approval
The data used in this study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by institutional review boards and independent ethics committees at participating 137 institutions.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All individual participants signed informed consent regarding publishing their data.
Availability of Data and Material
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Janssen, J.M., Damoiseaux, D., van Hasselt, J.G.C. et al. Semi-physiological Enriched Population Pharmacokinetic Modelling to Predict the Effects of Pregnancy on the Pharmacokinetics of Cytotoxic Drugs. Clin Pharmacokinet 62, 1157–1167 (2023). https://doi.org/10.1007/s40262-023-01263-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01263-1