Abstract
Introduction
Understanding the pharmacokinetics (PK) of antimicrobial drugs in pregnant women is crucial to provide effective and safe treatment. This study is part of a series that systematically reviews literature on the PK and analyzes if, based on the changed PK, evidence-based dosing regimens have been developed for adequate target attainment in pregnant women. This part focusses on antimicrobials other than penicillins and cephalosporins.
Methods
A literature search was conducted in PubMed according to the PRISMA guidelines. Search strategy, study selection, and data extraction were independently performed by two investigators. Studies were labeled as relevant when information on the PK of antimicrobial drugs in pregnant women was available. Extracted parameters included bioavailability for oral drugs, volume of distribution (Vd) and clearance (CL), trough and peak drug concentrations, time of maximum concentration, area under the curve and half-life, probability of target attainment, and minimal inhibitory concentration (MIC). In addition, if developed, evidence-based dosing regimens were also extracted.
Results
Of the 62 antimicrobials included in the search strategy, concentrations or PK data during pregnancy of 18 drugs were reported. Twenty-nine studies were included, of which three discussed aminoglycosides, one carbapenem, six quinolones, four glycopeptides, two rifamycines, one sulfonamide, five tuberculostatic drugs, and six others. Eleven out of 29 studies included information on both Vd and CL. For linezolid, gentamicin, tobramycin, and moxifloxacin, altered PK throughout pregnancy, especially in second and third trimester, has been reported. However, no target attainment was studied and no evidence-based dosing developed. On the other hand, the ability to reach adequate targets was assessed for vancomycin, clindamycin, rifampicin, rifapentine, ethambutol, pyrazinamide, and isoniazid. For the first six mentioned drugs, no dosage adaptations during pregnancy seem to be needed. Studies on isoniazid provide contradictory results.
Conclusion
This systematic literature review shows that a very limited number of studies have been performed on the PK of antimicrobials drugs—other than cephalosporins and penicillins—in pregnant women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
During pregnancy, several mechanistic and pathophysiological changes occur. Immunologic alterations occurring in pregnancy may help to explain the altered severity of and susceptibility to infectious diseases during pregnancy. |
This systematic review provides a complete and comprehensive overview of all studies regarding pharmacokinetics (PK), target attainment, and evidence-based dosing regimens of antimicrobial drugs—other than penicillin and cephalosporins—throughout pregnancy. |
This systematic literature review shows that current knowledge gaps include almost all antimicrobial drugs, other than penicillins and cephalosporins, that lack data altogether in this patient population. |
With this literature review we hope to stimulate other researchers to fill in the missing gaps by providing both PK data as well as dosing guidance for clinical implementation. Optimization of antibiotic treatment is vital for this vulnerable population. |
1 Introduction
Because of several mechanistic and pathophysiological changes (e.g., decrease in respiratory volumes and urinary stasis due to an enlarging uterus), pregnant women could be more severely affected by bacterial infections than non-pregnant women [1]. Immunologic alterations during pregnancy may help to explain the altered severity of and susceptibility to infectious diseases during pregnancy. As pregnancy progresses, hormone levels change dramatically. Estradiol can enhance several aspects of the innate immunity and both cell-mediated and humoral adaptive immune response. Progesterone can suppress the maternal immune response and alter the balance between T-helper (Th)1 and Th2 cell response [2]. It is estimated that during pregnancy, one third of pregnant women receive an anti-microbial drug [3]. Because of risk for the fetus, it is important that infections are adequately treated at an early stage to prevent complications [1].
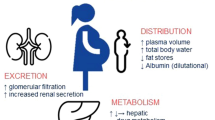
The anatomical and physiological changes that occur during pregnancy comprise a decrease in intestinal motility, an increase in total body water and an increase in glomerular filtration rate [4]. These changes can directly influence volume of distribution (Vd) and clearance (CL) of drugs. It is therefore likely that the pharmacokinetics (PK) of antimicrobial drugs change during pregnancy [5]. The above-mentioned physiological changes may lead to either subtherapeutic or toxic drug concentrations in the mother. The latter, especially, could potentially lead to toxic drug concentrations in the fetus. As a result, dosage adaptations are often necessary [4]. Currently, pregnant women are often administered the same dose as non-pregnant women [5]. Understanding the PK and how to reach the pharmacodynamic (PD) targets of antimicrobial drugs in pregnant women is essential to obtain evidence-based dosing regimens and to provide the most effective and safe treatment. While the PK for antimicrobial drugs have been extensively studied in non-pregnant adult populations, knowledge of PK and the ability to reach adequate target attainment of antimicrobial drugs in pregnant women is limited.
In separate contributions, we have reviewed the effect of pregnancy on the PK of penicillins [6] and cephalosporins (in preparation). This systematic literature review aims to describe PK and exposure as well as target attainment of antimicrobial drugs other than penicillins and cephalosporins throughout pregnancy (and when possible, compared with non-pregnant women). The effect of the antimicrobial drug on the fetus is outside the scope of this review. Furthermore, in this study it will be analysed if, based on the changed PK, evidence-based dosing regimens have already been developed for adequate target attainment in pregnant women.
2 Methods
2.1 Search Strategy
This systematic literature review is performed in accordance with the PRISMA guidelines of 2020 [7]. A search was conducted using PubMed on 1 September 2021 and updated on 28 August 2022 for a selection of antimicrobials. The following antimicrobial drugs were included in the search: aminoglycosides (amikacin, framycetin, gentamicin, neomycin, paromomycin, streptomycin, tobramycin); carbapenems (ertapenem, imipenem, and meropenem); quinolones (ciprofloxacin, levofloxacin, moxifloxacin, norfloxacin, ofloxacin); glycopeptides (dalbavancin, oritavancin, teicoplanin, vancomycin); macrolides (azithromycin, clarithromycin, erythromycin); polypeptides (bacitracin, colistin, gramicidin, polymyxin B); rifamycines (rifabutin, rifampicin, rifaximin); sulfonamides (sulfadiazine, sulfamethoxazole, sulphametrole, sulphapyridine); tetracyclines (demeclocycline, doxycycline, eravacycline, minocycline, oxytetracycline, tetracycline, tigecycline); tuberculostatic drugs (bedaquiline, cycloserine, delamanid, ethambutol, isoniazid, para-aminosalicylic acid, prothionamide, pyrazinamide); and others (including dapsone, clofazimine, aztreonam, chloramphenicol, daptomycin, fidaxomicin, fosfomycin, fusidic acid, linezolid, methenamine, mupirocin, nitrofurantoin, tedizolid, trimethoprim). Three domains, referring to the PICO elements of the research question (‘pharmacokinetics’, ‘pregnancy’ and ‘antimicrobial drugs’) were used in the search (Table 1).
For each antimicrobial drug we performed a separate search, indicating that overall 62 unique searches were conducted. Keywords were allocated to these domains and as many relevant synonyms for each keyword as possible were included in the search. Whenever possible, keywords were converted to corresponding MeSH terms and/or title/abstract terms. In the final search, both MeSH terms and keywords searched for in the title and abstract were included The MeSH and title/abstract terms used for each search are shown in Supplementary Data File Table 2 (see electronic supplementary material [ESM]). Additionally, studies were identified through reference checks of the included studies. Search results were stored in Microsoft Excel (version 16.59).
2.2 Inclusion Criteria
We included studies comparing PK of the drug of interest in pregnant women with that of non-pregnant or postpartum women, to accurately determine the potential influence of pregnancy. However, a full separate search on PK data for antimicrobial drugs in non-pregnant and postpartum women was not performed, as differences in PK results could be caused by different study methodologies. Thus, only non-pregnant and/or postpartum women data, if reported within the context of a study with pregnant women, were found eligible for this literature review. Comparison of PK parameters between non-pregnant/postpartum and pregnant women were made as follows: the percentage changes were calculated between those two groups and reported in the results section. When multiple studies reported PK data, the lowest and highest percentage changes between non-pregnant and pregnant women within all those studies were reported in the results section. The following types of studies were included in this literature review: prospective or retrospective cohort studies, randomized control studies, case-control studies, and case-series. We did not include reviews and physiological-based PK studies. Only studies performed in humans were included and studies written in the English language. All dosage forms (intravenous, intramuscular, and oral) were included in this study, as locally acting drugs can be reabsorbed to some extent (e.g., miconazole) [8]. If at least one PK parameter was investigated in pregnant women, the study was included. This literature review only focusses on PK-related endpoints and does not include efficacy studies. However, for antimicrobial drugs other than penicillins and cephalosporins it is known that efficacy is supported by reaching the PK/PD free-drug concentrations above the minimal inhibitory concentration (MIC) at the site of infection (fT > MIC), peak concentration over MIC (fCmax/MIC) or area under the curve (AUC) over MIC (fAUC/MIC) [9]. Furthermore, this literature review is limited to pregnant women, without including additional PK data from literature on the fetus.
2.3 Study Selection
During the initial selection, duplicate articles were excluded and titles and abstracts were screened for relevance to the study. Full texts of articles were obtained. Studies not meeting the study aim and inclusion criteria were excluded. The search strategy and study selection were separately performed by two investigators (FG and PM). Obtained results were discussed. In case of disagreement, a third author (DT) was consulted.
2.4 Data Extraction
Data extraction from all studies included was performed by two separate investigators (FG and PM).
In case of disagreement, a third author (DT) was consulted. Study and population characteristics such as study design, population number, age, weight (and BMI if reported), height, conditions, gestational age (GA), dosage form, and dose were identified and collected. The collected PK parameters of interest were bioavailability (F) for oral drugs, volume of distribution (Vd), and clearance (CL). Furthermore, exposure parameters such as trough (Cmin) and peak (Cmax) drug concentrations, time of maximum concentration (tmax), area under the curve (AUC), and half-life (t½) were collected. Collected PD parameters included probability of target attainment (PTA) and MIC. Finally, it was investigated if, based on the potentially changed PK/PD, adapted dosages were advised by the studies for adequate target attainment. Data was, when possible, stratified over six different pregnancy-related conditions of the patient populations: non-pregnant; first, second, third trimester of pregnancy, intrapartum and postpartum. All data extracted from the included studies was stored in Microsoft Excel (version 16.59).
3 Results
3.1 Study Selection and Data Extraction
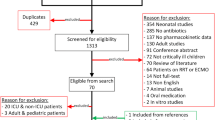
A detailed overview of the study selection is presented in the PRISMA flow diagram in Fig. 1. Twenty-nine studies were included in this systematic literature review, providing data for 8 of the 11 originally selected antimicrobial drug classes. The PK and exposure of the antimicrobial drugs are presented in alphabetical order of antimicrobial class and drugs within this class. Subsequently, the antimicrobial drugs are presented in the result section according to the ADME (absorption [if applicable], distribution, metabolism, elimination) sequence, followed by the obtained target, PTA, and evidence-based dosages if provided.
3.2 Aminoglycosides
No PK or exposure studies could be found for amikacin, framycetin, neomycin, paromomycin, and streptomycin in pregnant women.
3.2.1 Gentamicin
One prospective cohort study of 18 third trimester pregnant women and four non-pregnant women reported on gentamicin PK and exposure [10]. All women were scheduled for a caesarean section or a gynecological surgery under general anesthesia and received gentamicin 4 mg/kg intravenously over 2–3 minutes, 10 minutes prior to surgical incision [10]. The study characteristics including the PK parameters of pregnant women (if possible, in comparison with non-pregnant women) from the included studies are reported in Table 2. When focusing on the distribution, a 39% higher Cmax was reached in pregnant compared with non-pregnant women. Furthermore, Vd was decreased by 24% in pregnant women. The CL was minimally increased by 5% during pregnancy. No p values were reported, except for the change in the elimination constant. This parameter was significantly increased in pregnant women compared with non-pregnant women (0.4127 ± 0.0736/h vs 0.3198 ± 0.0943/h; p < 0.05) [10].
In summary, it is plausible that the PK and exposure of gentamicin changed during pregnancy based on the limited data; however, more robust studies are needed. Regardless of the change in PK and exposure parameters during pregnancy, no statements on target attainment or suggestions for the starting dose were reported.
3.2.2 Tobramycin
Two papers investigated the PK and exposure of tobramycin during pregnancy, one being a prospective cohort study and one a case report with 18 pregnant women in their second or third trimester. All pregnant women were admitted to the hospital for an underlying disease demanding tobramycin treatment. In the cohort study, the women received tobramycin 2.5 mg/kg once daily intravenously over 15 minutes for 7 ± 1 days, while in the case report tobramycin 2 mg/kg was administered intravenously once over 10 minutes, 5.6 hours before caesarean (Table 2) [11, 12]. PK parameters were reported in the cohort study, in contrast with the case report. Maximum concentrations (Cmax) were slightly higher (7%) in third-trimester pregnant women than in second-trimester pregnant women. In addition, it was observed that Vd was comparable between second-and third-trimester pregnant women [11]. This result was also supported by the case report [12]. As for the elimination, AUC was increased by 22% during the third trimester compared with the second trimester. CL was significantly higher with 21% (no p-value reported) in the second trimester compared with the third trimester [11, 12], explaining the difference in AUC.
In summary, the PK and exposure of tobramycin seem to change throughout pregnancy. No PTA was reported. The authors recommended accurate therapeutic drug monitoring during pregnancy [11, 12], but did not provide evidence-based starting dose advice.
3.3 Carbapenems
No studies were found that investigated the PK or exposure for ertapenem and meropenem in pregnant women.
3.3.1 Imipenem
Only one paper investigated the PK and exposure of imipenem during pregnancy. This prospective cohort study included a total of 20 subjects, of whom seven were in their first trimester, seven in their third trimester and six were not pregnant. Imipenem was given in a single intravenous dose of 500 mg (imipenem-cilastatin 1:1) as infusion over 20 minutes (Table 3) [13]. When focusing on the distribution, Cmax, measured immediately after infusion, and plasma concentrations 2 hours after administration, were significantly decreased by 65% (p < 0.05) in first and third trimester pregnant women in comparison with non-pregnant women. Vd was significantly increased by 60% and 65% for first (p < 0.005) and third trimester (p < 0.05) compared with non-pregnant women, respectively. Furthermore, the AUC was also significantly decreased (p < 0.05) during pregnancy by 44% and 58% for first and third trimester compared with non-pregnant women, respectively. The total CL was significantly increased (p < 0.05) in the pregnant patients (52% and 65% during first and third trimester compared with non-pregnant women) [13].
In summary, although only one study has been performed, there are indications that the PK or exposure of imipenem significantly changes during pregnancy. PTA was not reported and no recommendation for evidence-based dosing was provided.
3.4 Quinolones
No studies were found that investigated the PK or exposure for norfloxacin in pregnant women.
3.4.1 Ciprofloxacin, Levofloxacin, and Ofloxacin
One prospective cohort study reported on the PK and exposure of ciprofloxacin and one on the PK and exposure of ofloxacin during the second trimester of pregnancy [14]. In each study, 20 pregnant women were included with fetuses affected by beta-thalassemia major undergoing termination of gestation. Both ciprofloxacin (200 mg) and ofloxacin (400 mg) were administered every 12 hours intravenously (Table 4). In addition, only one prospective cohort study studied the PK and exposure during pregnancy for levofloxacin [15]. Levofloxacin 500 mg was given intravenously over 60 minutes to 12 pregnant women scheduled to undergo caesarean section for obstetric indications. Only maternal concentrations were reported in the above-mentioned studies (Table 4).
In summary, no conclusion can be made about PK or exposure changes for ciprofloxacin, ofloxacin, and levofloxacin in pregnant women.
3.4.2 Moxifloxacin
Three studies, two prospective cohort studies and one case report, reported on the PK and exposure of moxifloxacin during pregnancy [15,16,17]. The prospective cohort study by Nemutlu et al. [17] included nine non-pregnant women and six pregnant women scheduled for caesarean section. Both groups received moxifloxacin 400 mg in a single dose intravenously over 20 minutes, with completion of infusion 30 minutes prior to surgical incision. Ten pregnant women scheduled for caesarean section were included in the prospective study by Ozyuncu et al. [15] receiving moxifloxacin 400 mg in a single dose intravenously over 60 minutes, with completion of infusion 20–25 minutes before surgical incision. The case report by van Kampenhout et al. [16] followed a pregnant woman with tuberculosis from the second trimester to 18 weeks postpartum. She received moxifloxacin 400 mg orally once a day (Table 4). The study of Ozyuncu et al. [15] only reported maternal concentrations and the case report by van Kampenhout et al. [16] only reported the AUC. As for the distribution-related parameters, Nemutlu et al. [17] reported a 65% decrease in Cmax during pregnancy. The Vd seemed to be increased during pregnancy by 70%. Furthermore, the AUC was decreased at delivery by 80% compared with non-pregnant women. No p-values were reported by Nemutlu et al. [17]. Contradictory results on AUC were reported in the case report [16], in which overall AUC did not change throughout pregnancy. The t½ seemed to be decreased by 37% at delivery compared with non-pregnant women (p-value not reported) [17].
In summary, based on the limited results, it seems that the PK and exposure of moxifloxacin changed during pregnancy compared with non-pregnant women. However, PTA and dose adjustments were not reported.
3.5 Glycopeptides
No PK or exposure studies could be found for dalbavancin, oritavancin, or teicoplanin throughout pregnancy.
3.5.1 Vancomycin
Four papers were found on PK and exposure of vancomycin during pregnancy [18,19,20,21]. Three were prospective cohort studies and one study was a case report (Table 5) [18]. In total, 86 pregnant women in labor were included [18,19,20,21]. Dosages and dosing intervals varied for these studies. Bourget et al. [18] administered vancomycin 15 mg/kg intravenously every 12 hours for 13 days. Laiprasert et al. [19] used a single dose of 1 g intravenously 6 hours before delivery. The study by Onwuchuruba et al. [20] included three dosing regimens; 1 g intravenously every 12 hours, 15 mg/kg intravenously every 12 hours, and 20 mg/kg intravenously every 8 hours. Towers and Weit [21] also administered vancomycin 20 mg/kg intravenously every 8 hours. Only the case report by Bourget et al. reported PK parameters [18]. No comparison with non-pregnant women was made. The other studies [19,20,21] only reported maternal serum levels.
In summary, based on these papers, the changes in PK or exposure of vancomycin during pregnancy cannot be determined. However, the studies from Onwuchuruba et al. [20] and Towers and Weit [21] both showed that a dosing regimen of 20 mg/kg intravenously every 8 hours (with a maximum individual dose not exceeding 2 g) before delivery resulted in therapeutic serum vancomycin levels in more than 80% of the mothers.
3.6 Macrolides
No studies were found that investigated the PK or exposure of the macrolides (azithromycin, clarithromycin, erythromycin) in pregnant women.
3.7 Polypeptides
No studies were found that investigated the PK or exposure of the polypeptides (bacitracin, colistin, gramicidin, polymyxin B) throughout pregnancy.
3.8 Rifamycines
No studies were found that investigated the PK or exposure of rifabutin and rifaximin in pregnancy.
3.8.1 Rifampicin
For the PK and exposure of rifampicin during pregnancy, one prospective cohort study with 33 HIV-infected pregnant women was conducted. Rifampin was given once daily orally in a fixed-dose combination tablet (Rifafour® or Rifinah®) at a dose of ~10 mg/kg. Most participants received 600 mg daily. Twenty samples were collected during the third trimester, four during delivery and 24 postpartum (Table 6) [22]. As for the distribution, Vd was used for weight-based allometric scaling of the PK model. No differences between pregnant and non-pregnant women were reported. The elimination of rifampicin seemed to be affected by pregnancy. CL/F was significantly decreased by 14% during pregnancy compared with postpartum (p = 0.026). However, the model-estimated Cmax was similar during pregnancy and postpartum. Furthermore, the observed proportion of women achieving the target Cmax (≥ 8 mg/L) was very similar during pregnancy (54%) versus postpartum (58%). The model-estimated AUC increased slightly in pregnant women [22].
In summary, this study suggests that although CL/F of rifampicin is decreased in pregnant HIV-infected women, this appears to only modestly increase the rifampicin concentration and thus the rifampicin exposure. Therefore, no dose adjustment of rifampicin for HIV-infected women seems necessary during pregnancy.
3.8.2 Rifapentine
One study reported on the PK and exposure of rifapentine during pregnancy. This was a phase I/II trial including 50 pregnant women with indications for tuberculosis prophylaxis. Twenty-five women received rifapentine in the second trimester, of which ten were HIV positive and 25 women received the drug in the third trimester, of which ten were also HIV positive. In this paper, isoniazid was also administered and studied for PK (see tuberculostatic drugs). Rifapentine was given at a dose of 900 mg/week orally in combination with isoniazid in a combination preparation named 3HP for 12 weeks (Table 6) [23]. The AUC for HIV-positive pregnant women was increased by 14% compared with postpartum women and this was 21–50% higher than for HIV-positive pregnant women. No p-value was reported for this increase in AUC. As for the CL/F of rifapentine, no significant difference was observed between second and third trimester. However, a 30% increase (p < 0.001) in CL/F of rifapentine was seen for HIV-positive women compared with HIV-negative women. Additionally, it was found that HIV-negative women had a 28% decrease in CL during pregnancy compared with postpartum (P < 0.001). Based solely on these results, it cannot be concluded whether the change in AUC and CL in HIV-positive pregnant women was caused by the effect of HIV or by the efavirenz-based antiretroviral regimen [23].
In summary, although CL/F is decreased in HIV-negative pregnant women compared with postpartum women, no dose adjustments are needed for pregnant women as all women achieved the same rifapentine exposure as postpartum women.
3.9 Sulfonamides
No studies were found that investigated the PK or exposure of sulfadiazines, sulfametrole, and sulfapyridine throughout pregnancy.
3.9.1 Sulfamethoxazole (With and Without Trimethoprim)
One prospective study was found that investigated PK and exposure of sulfamethoxazole, including 20 pregnant women in total, of which 13 received sulfamethoxazole and seven received sulfamethoxazole in combination with trimethoprim. Both drugs were administered orally; sulfamethoxazole 480 mg every 12 hours for 13 women in combination with trimethoprim 960 mg every 12 hours. All women were in the first or second trimester of pregnancy and were admitted for abortion or tubal ligation. Maternal serum levels of sulfamethoxazole and trimethoprim were measured (Table 7) [24].
In summary, it is not known if the PK or exposure of sulfamethoxazole and trimethoprim changed during pregnancy as there was no comparison for PK and exposure during different trimesters of pregnancy or with non-pregnant women. PTA and dose adjustments were not reported.
3.10 Tetracyclines
No studies were found that investigated the PK or exposure of tetracyclines (demeclocycline, doxycycline, eravacycline minocycline, oxytetracycline, tetracycline, and tigecycline) in pregnancy.
3.11 Tuberculostatic Drugs
No PK or exposure studies have been found for bedaquiline, cycloserine, delamanid, para-aminosalicylic acid, and prothionamide in pregnant women. It must be noted that other drugs like moxifloxacin, ofloxacin, and linezolid also belong to the tuberculostatic drugs. These are described in Sects. 3.4 and 3.12, respectively.
3.11.1 Ethambutol
One prospective cohort study reported on the PK and exposure during pregnancy. This study included 18 samples from pregnant women with HIV infection treated for tuberculosis, which were studied both in third trimester and/or at delivery and postpartum. Women received a calculated number of 275-mg tablets orally daily (Rifafour® and Rifinah® combination preparation), with the dose adjusted for weight to 15–25 mg/kg (Table 8) [25]. Both the Cmax and AUC of ethambutol were slightly, but not significantly, increased by 13% during pregnancy. As for Vd/F and CL/F, no significant differences were observed between pregnant and postpartum women. No p-values were reported in this study [25].
In summary, it is very likely that the PK or exposure of ethambutol does not change during pregnancy. This also suggests that pregnant women could be treated with the same dose of ethambutol as postpartum women.
3.11.2 Isoniazid
Three studies reported on the PK and exposure of isoniazid during pregnancy [25]. Two studies were prospective cohort studies and one was a phase I/II trial. The first prospective study by Abdelwahab et al. [25] included a total of 29 samples, 18 during third trimester, 3 during delivery, and 8 postpartum. All women were pregnant and HIV positive, treated for tuberculosis by administering a calculated number of 75-mg tablets orally daily (Rifafour® and Rifinah® combination preparation), with the dose adjusted for weight to 4–6 mg/kg. The other prospective study of Gausi et al. [26] included 847 HIV-positive pregnant women. They received 300 mg orally daily for 28 weeks (immediately during pregnancy or starting at 12 weeks postpartum). A total of 420 levels were measured during second or third trimester and 637 levels were measured postpartum. In 210 patients, levels were measured both during pregnancy and postpartum. In the phase I/II trial by Mathad et al. [23], 50 pregnant women treated for tuberculosis were included. The women received isoniazid 900 mg weekly orally in combination with rifapentine in a combination preparation named 3HP for 12 weeks. Twenty-five women were in the second trimester, of whom 10 were HIV positive. Twenty-five women were in the third trimester, of whom ten were also HIV positive (Table 8).
The study by Mathad et al. reported no significant differences in model-estimated Cmax and AUC between second- and third-trimester pregnant and postpartum women [23]. The model-estimated Cmax was slightly, but non-significant (p-value was not reported), higher (2–14%) in two studies comparing postpartum with second/third trimester [25, 26]. Abdelwahab et al. [25] reported a model-estimated non-significant increase of 27% in AUC (no p-value reported) during the third trimester compared with the postpartum phase. On the contrary, Gausi et al. [26] found a decrease in AUC of 23% (no p-value reported) when comparing the second/third trimester with postpartum. However, both studies also showed a wide range of AUC values. The studies of Abdelwahab et al. [25] and Mathad et al. [23] reported no differences in Vd/F and CL/F between pregnant and postpartum women. Contradictory results were reported by Gausi et al. [26], who reported a 26% increase (p < 0.001) in CL/F during pregnancy.
In summary, there are conflicting results concerning PK and exposure changes of isoniazid during pregnancy. Abdelwahab et al. [25] concluded that the PK and exposure of isoniazid were, overall, not affected by pregnancy. However, the largest study by Gausi et al. [26] did conclude a reduction in isoniazid exposure during pregnancy and postpartum. The effect of PK and exposure on dosing of isoniazid is not stated and should be studied further.
3.11.3 Pyrazinamide
One prospective cohort study reported on the PK and exposure of pyrazinamide during pregnancy. This study included a total of 18 samples, all from pregnant women with HIV infection treated for tuberculosis. Pyrazinamide was administered as 400-mg tablets orally daily (Rifafour® and Rifinah® combination preparation), with the dose adjusted for weight to 20–30 mg/kg. Thirteen samples were taken during the third trimester, two while the patient was in labor, and three samples were collected postpartum (Table 8) [25]. The PK parameters for pyrazinamide showed that AUC and Cmax could be slightly decreased during pregnancy, by 2.8% and 3.9%, respectively. The Vd and CL was not different between pregnant and postpartum women. No p-values were reported [25].
In summary, it seems that the PK and exposure of pyrazinamide do not change during pregnancy and that therefore the dose can remain the same as for non-pregnant women.
3.12 Other Antimicrobial Drugs
No PK or exposure studies have been found that were performed in pregnant women for dapsone, clofazimine, aztreonam, daptomycin, fidaxomicin, fosfomycin, fusidic acid, methenamine, mupirocin, and tedizolid.
3.12.1 Chloramphenicol
One paper reported on the PK and exposure of chloramphenicol in which chloramphenicol 100 mg was vaginally administered daily in tablet form for 7 days, indicated for bacterial vaginosis in 37 pregnant women [27] (Table 9). This study reported maternal plasma levels that ranged from 0.043 × 10-3 to 0.0731 mg/L. This remained under the therapeutic concentration of 5.0–20.0 mg/L [27]. Due to these limited findings, no conclusions can be drawn regarding whether the PK or exposure of chloramphenicol changes during pregnancy.
3.12.2 Clindamycin
Three prospective cohort studies with a total of 44 subjects investigated the PK and exposure of clindamycin [28,29,30]. Fourteen subjects received clindamycin 450 mg orally after 8 or more hours of fasting as prophylaxis right before undergoing abortion [29]. The other 30 subjects received clindamycin intravenously as prevention for diagnosed Group B Streptococcus (GBS) (900 mg/kg every 8 h) or for prevention of endocarditis (600 mg every 6 h). The studies were all performed during different stages of pregnancy ranging from the first trimester to delivery (Table 9) [28,29,30]. From the maternal serum levels found, it can be seen that in the study of Wear et al., all maternal levels were above the target of >0.5 mg/L [30]. This concentration is also exceeded in the other two studies [28, 29]. Further PK or exposure parameters were not reported. Only the study by Muller et al. [28] studied other PK parameters. The Vd/F found in pregnant women in the third trimester reported by Muller et al. was 6.32∙103 L at steady state [28]. The elimination of clindamycin was not compared with non-pregnant women in this study, but the CL (10.0 L/h) was lower compared with values found in literature (19.8–26.4 L/h) [28].
Overall, it cannot be assessed if PK and exposure of clindamycin changed during pregnancy, as no comparison has been made with non-pregnant women. However, the study by Muller et al. [28] specified that an MIC of 0.5 mg/L can be reached when the protein binding is not higher than 65% after a dose of 900 mg every 8 hours intravenously. This finding was also supported by Wear et al. [30]. It should be noted that the possibility exists that protein binding is higher, and that the current dosing regimen is not adequate to protect all neonates from GBS [28].
3.12.3 Linezolid
One case report was found of a 25-year-old pregnant woman with tuberculosis, receiving linezolid 300 mg twice daily starting at 20 weeks’ gestational age. This treatment was stopped at 5 months postpartum (Table 9) [16]. Only the AUC is reported in this case report. The exposure was decreased during the second (by 76%) and third (by 48%) trimester compared with postpartum. In this case report, there was evidence of a change in the PK and exposure of linezolid for this single patient; however, further studies are needed to confirm this result. No conclusions on PTA and dose adjustments for pregnant women are provided.
3.12.4 Nitrofurantoin
One prospective cohort study of 17 pregnant women in labor reported on the PK and exposure of nitrofurantoin. Nitrofurantoin 90 mg was given intravenously over 30 minutes [31]. It has to be noted that currently the intravenous formulation is no longer available. This study did not report any PK and exposure parameters besides the half-life measured in 11 women and maternal serum levels (Table 9). Any conclusive changes in PK or exposure during pregnancy cannot be derived from this study.
4 Discussion
Antimicrobial drugs are some of the most prescribed drugs for pregnant women [4]. Based on this systematic literature review we can conclude that a very limited number of studies have been performed on the PK of antimicrobials drugs—other than penicillins [6] and cephalosporins (data unpublished)—in pregnant women. Therefore, only limited interpretations are possible. Of the 62 drugs included in the search strategy of this systematic review, PK and exposure parameters during pregnancy of only 18 drugs were reported; this means that 71% of the antimicrobial drugs—other than penicillins and cephalosporins—have not been studied during pregnancy. For 11 out of these 18 drugs, primary PK parameters such as Vd and/or CL were reported. The limited PK studies performed with these antimicrobial drugs in pregnant women show that overall PK is altered, especially during the second and third trimester compared with non-pregnant or postpartum women, resulting in lower exposure. For linezolid, gentamicin, tobramycin, and moxifloxacin, altered PK throughout pregnancy, especially in second and third trimester, has been reported. However, no target attainment was studied and no evidence-based dosing developed. On the other hand, the ability to reach adequate targets and assess the need for evidence-based dosing was assessed for vancomycin, clindamycin, rifampicin, rifapentine, ethambutol, pyrazinamide, and isoniazid. For the first six of these drugs, no dosage adaptations during pregnancy seem to be needed. Studies on isoniazid provide contradictory results. It is not surprising that very limited data is available in the pregnant population on drugs outside of the penicillin and cephalosporin classes. This is due to the fact that these classes are frequently prescribed and, based on human and animal studies, appear to be safe [32]. For many of the non-penicillin and non-cephalosporin drugs, there is animal data suggesting fetal toxicity. This data does not support the use of these drugs in pregnant women and hence there is very limited human data. To guide clinicians, in Table 10 we have provided recommendations for the use of these drugs during pregnancy, based on results from human and animal studies, and Briggs’ ‘Drugs in Pregnancy and Lactation’ was used as major source to formulate these recommendations [32]. For example, aminoglycosides such as amikacin, gentamicin, tobramycin, and streptomycin are generally recommended to be avoided due to their teratogenic effects (e.g., ototoxicity and nephrotoxicity) reported from human or animal studies (Table 10). PK data, and even safety data, for aminoglycosides are limited as for this class the risk often outweighs the benefit to the fetus/mother when other antimicrobials are available. As a consequence, the possibility of studying these drugs in pregnant women is limited, as is supported by the results obtained from our systematic review. Both amikacin and framycetin PK have not been studied during pregnancy, while limited PK studies (N = 3) are available for tobramycin and gentamicin during pregnancy [10,11,12]. For neomycin, it has to be noted that this is mainly topically administered. Paromomycin can be studied in pregnant women as no fetal toxicity has been reported (Table 10). This probably relies on the fact that it is applied orally and overall absorption is poor with almost 100% recovery in the feces. Therefore, it is likely to have little to no effect on the fetus [33]. For the carbapenems, such as ertapenem, some fetal abnormalities such as decreased fetal weight and decreases in average number of ossified sacrocaudal vertebrae (Table 10) have been reported in animal studies; this hinders the use of ertapenem in humans. This is also supported by the results from our systematic review. However, for both meropenem and imipenem, limited human and animal data suggest low risk, indicating that PK of those drugs can be further investigated. For all quinolones, strict contraindications are provided for use in pregnant women, mainly due to the fact that cartilage and joint abnormalities are reported in animal studies in the earlier stages of pregnancy (Table 10). Thus, PK data is limited, but PK studies performed with quinolones (N = 6) overall included pregnant women in later stages of pregnancy such as at caesarean section/delivery [15,16,17], showing no fetal risks after maternal quinolone exposure. For glycopeptides, dalbavancin seems to be teratogenic based on animal studies (decreased fetal maturation, reduced fertility). For oritavancin and teicoplanin, no data from human and animal studies is presently available in Briggs’ ‘Drugs in Pregnancy and Lactation’ [32]. It has to be noted that dalbavancin and oritavancin have weekly or longer dosing intervals, so studies are very challenging in any population. This is also supported by our systematic review as no PK studies could be found for these drugs. For the glycopeptide vancomycin, no evidence for teratogenicity or fetotoxicity from human and animal studies has been reported, therefore vancomycin is considered safe for all pregnancy states (Table 10). Although four PK studies on vancomycin during the last trimester of pregnancy reported similar PK and exposure in late-trimester pregnant and non-pregnant women, indications are still present to further investigate the PK of this drug in earlier stages of pregnancy as PK data is still absent in those trimesters. For macrolides, erythromycin is the only macrolide recommended in the clinical pregnant population; but data are controversial for azithromycin and clarithromycin. Both have been linked to cardiovascular anomalies (Table 10). It is surprising that no PK data for these three macrolides could be found for pregnant women. Therefore, PK (and safety data) for mother/fetus should be collected for these three macrolides. For polypeptides, both bacitracin and gramicidin are used topically and thus no PK studies are available. For colistin and polymyxin B, no data from human and animal studies seems to be available in the current edition of Briggs’ ‘Drugs in Pregnancy and Lactation’ (Table 10). For the rifamycines, rifaximin and rifabutin have a low to moderate risk based on animal data (Table 10) and thus both are not used in clinical practice. For rifapentine, teratogenic effects mainly occur in early stages of pregnancy (Table 10). Therefore, caution is needed in prescribing rifapentine in early stages of pregnancy. Currently, one PK study has been performed for the PK of rifapentine in the third trimester showing similar PK and exposure compared with post-partum. Rifampicin, however, is reported to be safe in pregnancy based on human and animal studies (Table 10) and one PK study reported similar PK and exposure in late-trimester pregnant compared with postpartum women. Thus, PK (and safety information for the fetus) should be further studied in earlier stages of pregnancy. For sulfonamides, no distinction in risk was made between the various sulfonamides within this specific class. Data from both human and animal studies suggest a risk, especially in the third trimester of pregnancy (Table 10).It has to be noted that one prospective study has been found investigating sulfamethoxazole PK in first- and second-trimester pregnant women undergoing termination of pregnancy [24]. Also, for tetracyclines, no distinction in risk was made between the various tetracyclines within this specific class. All tetracyclines are contra-indicated in the second and third trimester of pregnancy; mainly because of fetal abnormalities, dental and bone issues, and maternal hepatotoxicity (Table 10). If a tetracycline is strictly indicated during pregnancy, doxycycline should be used only in the first trimester of pregnancy. These findings are in line with the results from our systematic literature review in which no studies were found that have investigated the PK or exposure of tetracyclines. For the tuberculostatic drugs, ethambutol is considered compatible in pregnancy as no teratogenic effects have been reported from human and animal studies (Table 10). Pyrazinamide and isoniazid are also labeled as compatible with pregnancy, although human studies have reported induction of chromosomal aberrations in human lymphocyte cell cultures and animal studies have reported embryonical effects, respectively, for these drugs (Table 9). However, it has to be noted that despite these safety issues, maternal benefit is more important compared with the embryo-fetal risk [32]. The PK of these three tuberculostatic drugs has been studied in more detail during pregnancy, including the ability to reach adequate target concentrations and the need to develop evidence-based dosing [22,23,24,25,26]. For all these drugs, except for isoniazid, exposure during pregnancy is unchanged, making dose adaptations unnecessary. For isoniazid, contradictory results on altered PK have been reported [23, 25] and more studies are needed. For the tuberculostatic drugs bedaquiline and cycloserine, low and moderate fetal risks are suggested due to limited available data from human and animal studies (Table 10) [32]. Based on human and animal studies, para-amino salicylic acid has been reported to have a risk for malformations, which again limits the possibility of studying the PK in pregnancy. This is also supported by the fact that no papers have been published on the PK in pregnant women. For delamanid and prothionamide, no data from human and animal studies are available in the current edition of Briggs’ ‘Drugs in Pregnancy and Lactation’ [32]. For the other antimicrobial drugs not belonging to a specific class, various labeling has been reported based on risk assessments in human and animal studies. Chloramphenicol, fosfomycin, methenamine, linezolid, and dapsone are labeled as compatible in pregnancy. No human data, but probably compatible in pregnancy, has been reported for mupirocin and tedizolid, while no human data but based on animal data, a fetal risk for clofazimine, aztreonam, daptomycin, and fidaxomicin is reported. Finally, for nitrofurantoin and trimethoprim, a fetal risk for use during pregnancy has been reported based on human or animal studies. In general, the PK of the drugs belonging to ‘other antimicrobial drugs’ (Sect. 3.12) is not investigated throughout pregnancy.
It has to be concluded that for most drugs, other than penicillins and cephalosporins, the predefined PK/PD relationships (fT > MIC, fCmax/MIC or fAUC/MIC) [9] currently used to develop evidence-based dosing regimens need to be further investigated, as these are mainly based on theoretical concepts and studies in critically ill patients [34]. Attainment of targets is a challenge to investigate. Target attainment mainly depends on the sensitivity of the micro-organisms in combination with the net exposure to the antibiotic. Thus, both bacterial sensitivity (MIC) and free concentration of the antibiotic need to be studied in pregnancy. It has to be noted that protein binding is a less well studied topic. A problem may be that microbial sensitivity can vary per area and dose recommendations established in high-income countries where these studies can be performed also need to be applicable in low- and middle-income countries where measurement of target attainment is less possible.
The limited number of PK studies found by performing this systematic literature search is a limitation. It has to be concluded that for most drugs, other than penicillins and cephalosporins, limited PK data is available. In addition, these PK studies have been performed with small numbers of pregnant and non-pregnant/postpartum patients. As a consequence, significant differences between these two populations are difficult to prove and bias can occur when interpreting the results. Focus should be on the primary PK parameters Vd and CL between pregnant and non-pregnant patients. Population-PK modelling in combination with simulations is a valuable tool to not only demonstrate clinically relevant differences in PK parameters but also to develop evidence-based dosing schemes to attain adequate targets in pregnant patients [5]. Another reason for the limited PK data being available is mainly due to maternal and/or fetal risks as there is no possibility of obtaining these data throughout pregnancy. This does not mean that no data is available at all. The possibility exists that drugs with a high safety risk will be incidentally used (e.g., when a patient is unaware that she is pregnant; has allergies for safer drugs, or no alternatives are available due to the nature of the infection). It is of the utmost importance to report these cases, including possible PK and safety data. When limited PK data is available, this information can serve as a basis to validate PK predictions from developed physiological-based PK (PBPK) models. Fetal–maternal PBPK (fm-PBPK) modeling is a pragmatic approach combining available compound models with a virtual maternal–fetal physiology model [35]. It can be an attractive tool to predict PK and increase knowledge on both maternal and fetal drug exposure, especially when combined with clinically collected short- and long-term safety data [35]. Another logical next step for future research is to study the PK of those antimicrobial drugs that are considered to be safe (such as meropenem, imipenem, erythromycin, azithromycin and clarithromycin), based on animal or human studies, in a larger heterogenous pregnant population to better describe exposure targets.
A final limitation of the studies found is the fact that only total concentrations of non-penicillin and non-cephalosporin drugs have been measured. It goes without saying that a changed protein binding will not affect the clearance of the free drug (as this usually stays the same), but the apparent clearance of the total drug. In future research, it is relevant to not only measure the total fractions, but also the free fraction of drugs with high (> 80%) protein binding being the active part.
5 Conclusion
This systematic literature overview shows that currently many knowledge gaps exist for almost all antimicrobial drugs, other than penicillins and cephalosporins, in the pregnant patient population. With this systematic review we hope to stimulate other researchers to fill these missing gaps by providing both PK data and dosing guidances for clinical implementation. Optimization of antibiotic treatment is vital for this vulnerable population.
References
Rac H, Gould AP, Eiland LS, Griffin B, McLaughlin M, Stover KR, et al. Common bacterial and viral infections: review of management in the pregnant patient. Ann Pharmacother. 2019;53(6):639–51.
Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370(23):2211–8.
Stojanova J, Arancibia M, Ghimire S, Sandaradura I. Understanding the pharmacokinetics of antibiotics in pregnancy: is there a role for therapeutic drug monitoring? A narrative review. Ther Drug Monit. 2022;44(1):50–64.
Pariente G, Leibson T, Caris A, Adams-Webber T, Ito S, Koren G. Pregnancy-associated changes in pharmacokinetics: a systematic review. PLoS med. 2016;13(11): e1002160.
Dallmann A, Mian P, van den Anker J, Allegaert K. Clinical pharmacokinetic studies in pregnant women and relevance of pharmacometric tools. Curr Pharm Des. 2019;25(5):483–95.
Hesse MR, Prins JR, Hooge MNLd, et al. Pharmacokinetics and target attainment of antimicrobial drugs throughout pregnancy: part I—penicillins. Clin Pharmacokinet. 2023. https://doi.org/10.1007/s40262-023-01211-z.
PRISMA Guideline. 2020. https://prisma-statement.org/PRISMAStatement/CitingAndUsingPRISMA. Accessed 01 Sep 2021
Pemberton MN, Oliver RJ, Theaker ED. Miconazole oral gel and drug interactions. Br Dent J. 2004;196(9):529–31.
Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother. 2005;55(5):601–7.
Popovíc J, Grujic Z, Sabo A. Influence of pregnancy on ceftriaxone, cefazolin and gentamicin pharmacokinetics in caesarean vs. non-pregnant sectioned women. J Clin Pharm Ther. 2007;32(6):595–602.
Bourget P, Fernandez H, Delouis C, Taburet AM. Pharmacokinetics of tobramycin in pregnant women. Safety and efficacy of a once-daily dose regimen. J Clin Pharm Ther. 1991;16(3):167–76.
Fernandez H, Bourgt P, Delouis C. Fetal levels of tobramycin following maternal administration. Obstet Gynecol. 1990;76(992):4.
Heikkila A, Renkonen OV, Erkkola R. Pharmacokinetics and transplacental passage of imipenem during pregnancy. Antimicrob Agents Chemther. 1992;36(12):2652–5.
Giamarellou H, Kolokythas E, Petrikkos G, Gazis J, Aravantinos D, Sfikakis P. Pharmacokinetics of three newer quinolones in pregnant and lactation women. Am J Med. 1989;87(5A):49S-51S.
Ozyuncu O, Nemutlu E, Katlan D, Kir S, Beksac M. Maternal and fetal blood levels of moxifloxacin, levofloxacin, cefepime and cefoperazone. Int J Antimicrob Agents. 2010;36(2):175–8.
Van Kampenhout E, Bolhuis MS, Alffenaar J-WC, Oswals LMA, Kerstjens HAM, et al. Pharmacokinetics of moxifloxacin and linezolid during and after pregnancy in a patient with multidrug-resistant tuberculosis. Eur Respir J. 2017;49(3):1601724.
Nemutlu E, Kir S, Eroglu H, Katlan D, Ozek A, Ozyuncu O, et al. Comparison of pharmacokinetic profles of moxifloxacin in Caesarean versus non-pregnant sectioned women by fully validated HPLC with fluorescence detection. Comb Chem High Throughput Screen. 2010;13(6):502–9.
Bourget P, Fernandez H, Delious C, Ribou F. Transplacental passage of vancomycin during the second trimester of pregnancy. Obstet Gynecol. 1991;78(5 pt 2):908–11.
Laiprasert J, Klein K, Mueller BA, Pearlman MD. Transplacental passage of vancomycin in noninfected term pregnant women. Obstet Gynecol. 2007;109(5):1105–10.
Onwuchuruba CN, Towers CV, Howard BC, Hennessy MD, Wolfe L, Brown MS. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210(4):352.e1-352.e4.
Towers CV, Weit B. J Transplacental passage of vancomycin. Matern Fetal Neonatal Med. 2018;31(8):1021–4.
Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, Msandiwa R, et al. Population pharmacokinetics of rifampicin in pregnant women with tuberculosis and HIV coinfection in Soweto, South Africa. Antimicrob Agents Chemother. 2015;60(3):1243–341.
Mathad JS, Savic R, Britto P, Jayachandran P, Wiesner L, Montepiedra G, et al. Pharmacokinetics and safety of 3 months of weekly rifapentine and isoniazid for tuberculosis prevention in pregnant women. Clin Infect Dis. 2022;74(9):1604–13.
Reid DW, Caille G, Kaufmann NR. Maternal and transplacental kinetics of trimethoprim and sulfamethoxazole, separately and in combination. Can Med Assoc J. 1975;14(112):67–72.
Abdelwahab MT, Leisegang R, Dolley KE, Mathat JS, Wiesner L, Mclleron H, et al. Population pharmacokinetics of isoniazid, pyrazinamide, ethambutol in pregnant south African women with tuberculosis and HIV. Antimicrob Agents Chemother. 2020;64(3):e01978-e2019.
Gausi K, Wiesner L, Norman J, Wallis CL, Onyango-Makumbi C, Chipato T, et al. Pharmacokinetics and drug-drug interactions of isoniazid and efavirenz in pregnant women living with HIV in high TB incidence settings: importance of genotyping. Clin Pharmacol Ther. 2021;109(4):1034–44.
Harauchi S, Osawa T, Kubono N, Itoh H, Naito T, Kawakami J. Transfer of vaginal chloramphenicol to circulating blood in pregnant women and its relationship with their maternal background and neonatal health. J Infect Chemother. 2017;23(7):4460451.
Muller AE, Mouton JW, Oostvogel PM, Dorr PJ, Voskuyl RA, DeJongh J, et al. Pharmacokinetics of clindamycin in pregnant women in the peripartum period. Antimicrob Agents Chemother. 2010;54(5):2175–81.
Philipson A, Sabath LD, Charles D. Transplacental passage of erythromycin and clindamycin. N engl J Med. 1973;288(23):1219–21.
Wear CD, Towers CV, Brown MS, Weitz B, Porter S, Wolfe L. Transplacental passage of clindamycin from mother to neonate. J Perinatol. 2016;36(11):960–1.
Perry JE, Leblanc AL. Transfer of nitrofurantoin across the human placenta. Tex Rep Biol Med. 1967;25(2):265–9.
Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2017.
Kreutner AK, Del Bene VE, Amstey MS. Giardiasis in pregnancy. Am J Obstet Gynecol. 1981;140(8):895–901.
De Waele J, Lipman J. Akova M, Vassetti M, Dimpoulos G, Kaukonon M et al. Risk factors for target non-attainment during empirical treatment with b-lactam antibiotics in critically ill patients. Intensive Care Med. 2014; 1340–51.
Brookhuis SAM, Allegaert K, Hanff LM, Lub-deHooge MN, Dallmann A, Mian P. Modelling tools to characterize acetaminophen pharmacokinetics in the pregnant population. Pharmaceutics. 2021;13(8):130.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest/competing interests
Femke Groen, Jelmer R. Prins, Marjolijn N. Lub-de Hooge, Rik L.J. Winter, Jos G.W. Kosterink, Daniel J. Touw and Paola Mian declare that they have no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Author contributions
FG contributed to the literature search, data extraction, data analysis and writing the initial draft of the manuscript. JRP, MNLDH, HLJW, JGWK contributed to study design and DJT contributed to conceptualization of the study, data analysis and reviewing the manuscript. PM contributed to the conceptualization of the study, literature search, data extraction, data analysis and writing and reviewing the manuscript.
Ethics approval
Not applicable.
Data availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Groen, F., Prins, J.R., Hooge, M.N.Ld. et al. The Pharmacokinetics and Target Attainment of Antimicrobial Drugs Throughout Pregnancy: Part III Non-penicillin and Non-cephalosporin Drugs. Clin Pharmacokinet 62, 399–434 (2023). https://doi.org/10.1007/s40262-023-01226-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01226-6