Abstract
Background and Objective
Pharmacokinetics (PK) are severely altered in pregnant women due to changes in volume of distribution (Vd) and/or drug clearance (CL), affecting target attainment of antibiotics in pregnant women. This review is part of a series that reviews literature on the description of PK and target attainment of antibiotics in pregnant women with specific focus on penicillins.
Methods
A systematic literature search was carried out in PubMed. Articles were labelled as relevant when information on PK of penicillins in pregnant women was available.
Results
Thirty-two relevant articles were included, 8 of which discussed amoxicillin (with and without clavulanic acid), 15 ampicillin, 4 benzylpenicillin, 1 phenoxymethylpenicillin, and 4 piperacillin (with and without tazobactam). No studies were found on pheneticillin and flucloxacillin in pregnant women. Ten out of 32 articles included information on both Vd and CL. During the second and third trimester of pregnancy, a higher CL and larger Vd was reported than in non-pregnant women and in pregnant women during first trimester. Reduced target attainment was described in second and third trimester pregnant women. Only 7 studies reported dosing advice, 4 of which were for amoxicillin.
Conclusion
The larger Vd and higher CL in second and third trimester pregnant women might warrant a higher dosage or shortening of the dosing interval of penicillins to increase target attainment. Studies frequently fail to provide dosing advice for pregnant women, even if the necessary PK information was available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pharmacokinetics (PK) are severely altered in pregnant women due to changes in volume of distribution (Vd) and/or drug clearance (CL), affecting target attainment of antibiotics in pregnant women. |
This systematic literature review aims to describe the PK and target attainment of penicillins in pregnant women. Volume of distribution and higher CL in second and third trimester pregnant women might warrant a higher dosage or shortening of the dosing interval of penicillins to increase target attainment. |
This systematic literature overview hopes to inspire researchers to fill these gaps, not only by publishing PK data, but also by providing evidence-based dosing regimens for implementation in the clinic, as this information is vital to optimise penicillin treatment in this special population. |
1 Introduction
During pregnancy, one-third of pregnant women receive an anti-microbial agent [1]. Because of physiological and mechanical changes during pregnancy, pregnant women could be more severely affected by specific bacterial infections than healthy non-pregnant women [2]. Therefore, some infections in pregnant women need to be treated in an early stage to prevent complications to mother and/or foetus. Antibiotics, such as penicillins, are a first choice of anti-infective therapy for many bacterial infections, especially in pregnancy, due to their safety [2].
During pregnancy, several changes in the female body occur, both anatomical and physiological. Examples of these changes during pregnancy are a decrease in intestinal motility, an increase in gastric acidity, and an increased glomerular filtration rate (GFR) (Fig. 1) [3, 4]. It is therefore likely that the pharmacokinetics (PK) of many drugs, including penicillins, are influenced during pregnancy [4]. The above-mentioned physiological changes and resulting changes in PK may lead to supra- or subtherapeutic drug concentrations in the mother. In addition, drug exposure could lead to toxic drug concentrations in the foetus as many drugs are either actively or passively transported over the placenta. As a result, dosage adaptations are often necessary [3]. Currently, pregnant women are often administered the same dose as non-pregnant women. Knowledge on altered PK parameters and the ability of reaching the pharmacodynamic (PD) targets is essential to obtain evidence-based dosing regimens in pregnant women.
This study aims to systematically review literature on the description of PK and target attainment of antibiotics in pregnant women. Furthermore, this study will analyse if, based on the reported changed PK/PD, evidence-based dosing regimens have already been developed to reach adequate target concentrations in pregnant women.
2 Methods
2.1 Search Strategy
This systematic literature review is performed in accordance by the PRISMA guidelines of 2020 [5]. The PRISMA checklist is shown in Supplemental File 1. A search was conducted using PubMed on September 1 2021, and updated on August 28 2022. For each included penicillin, a search was performed, with a combination of different terms: ‘drug name’, ‘pregnancy’ and ‘pharmacokinetic’. For the specific keywords and field codes per topic, see Table 1. The detailed search strategy is outlined in Supplemental File 2. References of included studies were checked for other relevant articles for this study.
2.2 Inclusion Criteria
This systematic review describes 7 penicillins: amoxicillin (with and without clavulanic acid), ampicillin, benzylpenicillin (penicillin G), pheneticillin, phenoxymethylpenicillin (penicillin V), flucloxacillin, piperacillin (with and without tazobactam). For each penicillin, a separate systematic search was conducted to find studies on the PK, the probability of target attainment, and development of evidence-based dosing regimens in pregnant women. Studies were included in which at least one PK parameter was stated in pregnant women. If possible, PK of the antibiotic was compared with that of non-pregnant women. All different dosage forms (intramuscular, intravenous, oral and vaginal) were included in this study, as locally acting drugs can be reabsorbed to some extent (e.g., miconazole) [6]. In addition, the following studies were included in this study when available: randomised controlled trial, non-randomised controlled trial, cohort study, case-control study or case-series study. A study in the form of a review was not included in this study and only studies performed in humans were included. In addition, only studies written in English were included. No year of publication restrictions were made. It has to be noted that this study only focusses on PK-related endpoints and does not include efficacy studies. However, for penicillins, it is known that efficacy is supported by reaching the PK/PD parameter time that the free fraction is above the MIC (fT>MIC) [7, 8]. Furthermore, this systematic literature review is limited to PK and target attainment in pregnant women, without including additional PK or safety data from literature on the foetus.
2.3 Study Selection
The title and abstract were screened for the relevance to this study. After initial selection, the full text of the articles was obtained. Studies not meeting the inclusion criteria were excluded. Two investigators (MH and PM) conducted the search strategy and study selection, separately. The obtained results were discussed and, in case of disagreement, a third author (DT) was consulted.
2.4 Data Extraction
After inclusion of a study, data were extracted. The data extraction was performed separately by two investigators (MH and PM) for all included studies. In case of disagreement, a third author (DT) was consulted. For each eligible article, data were systematically extracted and all extracted data were entered in a database using Microsoft Excel by one author (MH). Thereafter, the data in the table were checked by a second author (PM). The extracted study characteristics of interest were: study design, number of participants, trimester of pregnancy (type of medication [with dosage and dosage interval]). In addition, PK parameters per study were extracted. The collected PK parameters of interest were bioavailability (F) for oral drugs, volume of distribution (Vd) and clearance (CL). Further, exposure parameters such as trough (Cmin) and peak (Cmax) drug concentrations, time of maximum concentration (tmax), area under the curve (AUC) and half-life (t1/2) were collected. Pharmacodynamic parameters that were collected included the probability of target attainment (PTA), minimal inhibitory concentration (MIC), and (fT>MIC). Further study investigation advised if adapted evidence-based dosing regimens for adequate target attainment were based on potentially changed PK/PD. Comparison of PK parameters between non-pregnant/postpartum and pregnant women were made as follows: when only one study reported PK data in non-pregnant and pregnant women, the percentage changes were calculated between those two groups and reported in the result section. When multiple studies reported PK data, the lowest and highest percentage changes between non-pregnant and pregnant women within all those studies were reported in the result section.
3 Results
3.1 Study Selection and Data Extraction
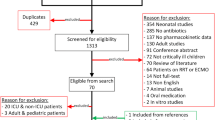
Study selection and data extraction for each included penicillin were performed. With the search strategy and based on title and abstract, 40 studies for amoxicillin (with and without clavulanic acid), 113 for ampicillin, 120 for benzylpenicillin, 0 for pheneticillin, 4 for phenoxymethylpenicillin, 3 for flucloxacillin and 12 for piperacillin (with and without tazobactam) were identified. After reading the full paper, 8 studies for amoxicillin (with and without clavulanic acid), 15 for ampicillin, 4 for benzylpenicillin, 0 for pheneticillin, 1 for phenoxymethylpenicillin, 0 for flucloxacillin and 4 studies for piperacillin (with and without tazobactam) were included. A detailed overview of the study selection is presented in the PRISMA flow diagram in Fig. 2.
An overview of the PK studies of penicillins during pregnancy is presented in the paragraphs below. Drugs are presented in alphabetical order. First, study characteristics of the individual penicillins are described. Second, PK and exposure are discussed according to the absorption, distribution, metabolism, elimination (ADME) sequence. Last, the probability of target attainment is described and, if available, evidence-based dosing regimens of the individual penicillins are discussed.
3.1.1 Amoxicillin (With or Without Clavulanic Acid)
A total of 8 studies on amoxicillin (with or without clavulanic acid) were included [9,10,11,12,13,14,15,16]. No PK studies reported the PK and exposure parameters of clavulanic acid. The patient characteristics of the included studies are listed in Table 2. The number of participants in the different studies ranged from 17 to 50. The mean age ranged from 29.0 to 31.8 years. The mean body weight throughout pregnancy ranged from 79.0 to 80.9 kg. The mean pre-pregnancy body weight and pre-pregnancy body mass index (BMI) ranged from 63.3 kg to 65.7 kg and from 23.1 to 24.0 kg/m2, respectively. Only one study reported all the different trimesters of pregnancy [9]. For the other 7 studies, gestational age ranged from 29.4 to 42.4 weeks. In four out of 8 studies, amoxicillin was administered intravenously, 3 orally and 1 vaginally.
The PK and exposure parameters of pregnant women (if possible, in comparison with non-pregnant women) of the included studies are summarised in Table 2. When focusing on the absorption phase, none of the 4 oral or vaginal studies reported absorption-related parameters, such as bioavailability (F) [9,10,11,12,13,14,15,16]. Only one study showed that Cmax decreased significantly in the second (21.8% points) and third trimester (33.3% points) of pregnancy compared to postpartum. The same study described the effects of pregnancy on the AUC [9]. At an oral dose of 500 mg, AUC decreased significantly, by 25.5% points and 27.0% points, respectively, in the second and third trimester of pregnancy compared to postpartum. Studies of Buckingham et al. and Zareba-Szczudlik et al. reported concentrations in maternal blood only [10, 14,15,16].
None of the 8 studies described the differences in Vd/F between pregnant and non-pregnant women. Only the intravenous (IV) studies performed by Muller et al. described the effects on Vd during the third trimester of pregnancy [11,12,13]. Results were obtained with the use of a developed population PK model. Two of the studies used a 3-compartment model and the other study described a 5-compartment model. The Vd in the central compartment ranged from 5.59 to 8.7 L. The first peripheral compartment ranged from 5.88 to 11.8 L. The second peripheral compartment ranged from 5.88 to 40.4 L [11,12,13].
Of all 8 studies, 4 reported elimination-related parameters (t1/2 and CL/F) [9, 11,12,13]. The reported mean CL/F in the different studies in pregnant women ranged from 19.7 to 35.5 L/h, corresponding with an increased CL/F with 86.4–123.3% compared to non-pregnant women. In comparison to the t1/2 3 months postpartum, the t1/2 was 18.8–31.3% shorter in pregnant than in non-pregnant women [9, 11, 12].
Of the 8 studies, 4 [9, 11,12,13] reported a desired target concentration and the probability of target attainment with the currently used dosing regimens of amoxicillin in pregnant women. The target varied per study and micro-organism (e.g., Group B Streptococcus, S. agalactiae, Table 2). Andrew et al. reported a probability of target attainment with different dosing simulations [9]. According to the studies of Muller et al. [11,12,13] no dosing adjustments for amoxicillin were necessary for pregnant women in the third trimester. These findings are partly supported by the study of Andrew et al. [9] that stated that dosing adjustments are not necessary for sensitive isolates. However, for less sensitive isolates, shortening the dosage interval is advised to reach the desired target concentration.
In summary, no direct comparison was made between pregnant and non-pregnant women. Little information is available on the effect of pregnancy on amoxicillin absorption. One study showed that AUC and Cmax during pregnancy were significantly lower than postpartum. No studies were performed to investigate the differences in distribution-related parameters between pregnant and non-pregnant women for amoxicillin, but CL/F is higher and the t1/2 is shorter in pregnant compared to non-pregnant women. The 4 studies that investigated target attainment reported that overall, no dosing adjustments for amoxicillin are necessary. However, for less sensitive isolates, shortening the dosage interval is advised to reach the desired target concentration.
3.1.2 Ampicillin
In total, 12 studies on ampicillin were included [10,11,12,13,14,15,16,17,18,19,20,21]. Table 3 provides the characteristics of the included PK studies. Among these studies, only one included pregnant women during their first trimester of pregnancy, 3 studies during the second trimester, 10 during their third trimester; from 2 studies the trimester of the included women was not reported. The number of participants in the different studies ranged from 3 to 40 women. The mean age of the patients included in the studies ranged from 20 to 40 years. Only one study [21] reported the BMI (mean 33.0 kg/m2). The other studies [10,11,12,13,14,15,16,17,18,19,20], reported weight which varied from 46.8 to 88.4 kg. Of the 12 studies, 2 investigated the PK of ampicillin after oral administration, 2 after intramuscular administration, 6 after intravenous administration and for 2 studies the route of administration was unknown. The PK and exposure parameters of ampicillin in pregnant women (where possible compared with non-pregnant women) of the included studies are summarised in Table 3.
Of all 12 studies, one reported the F [22]. The mean F in non-pregnant women was 2.5% points higher compared with pregnant women (45.6 vs 48.1%). Six studies reported the Cmax and Cmean. After oral administration, the Cmax values reached in non-pregnant women were significant higher than in pregnant women (difference 40.5% points) [22]. Creatsas et al. [23] also investigated the effects on Cmean, Cmax and tmax of intramuscular administration in pregnant women in their third trimester. The authors found a Cmax of 7.88 μg/mL in mother’s blood 1 h after administration. When focussing on the distribution, Chamberlain et al. [20] stated that the Cmax reached in pregnant women (trimester not specified) was 8.1% points higher than Cmax reached in non-pregnant women after IV administration [20]. Philipson et al. [22] concluded that the Cmax after IV administration is comparable in pregnant (all trimesters) to non-pregnant women (difference of 1.6% points). Bloom et al. [18] and Bray et al. [19] investigated the effects on Cmax of IV administration in pregnant women in their third trimester. Bloom et al. [18] found a Cmax in mother’s blood of 142 μg/mL, 3 min after infusion, where Bray et al. [19] found a mean Cmax of 26.5 μg/mL, directly after infusion.
The Vd/F of ampicillin was investigated in 4 studies [17, 20, 22, 24]. Of these 4 studies, 3 investigated the differences between pregnant and non-pregnant women. One study stated that the Vd in pregnant women is slightly lower (5.0% points) than in non-pregnant women [20]. Contradicting results were mentioned in another study; namely a 38.4%-point higher Vd in pregnant women than in non-pregnant women was reported [22]. Assael et al. [17] supported this increase (45.6%) in Vd/F in pregnant compared to non-pregnant women.
The studies of Assael et al. [17], Chamberlain et al. [20] and Philipson et al. [22] investigated the effects of pregnancy on the AUC, in comparison to non-pregnant women. Assael et al. [17] concluded that the AUC in pregnant women in their second and third trimester was 37.3% points lower compared with non-pregnant women [17]. The studies of Chamberlain et al. [20] and Philipson et al. [22] supported the decrease of AUC in pregnant women, with 20.7% points and 34.9% points, respectively. Explaining the difference in AUC, CL/F in pregnant women was significantly higher than in non-pregnant namely, 179.1% [17] 122.4% [20] and 155.6% [22].
The majority of the studies investigated ampicillin PK for Group B Streptococcus (GBS) prophylaxis. Overall, those studies reported that in 100% of the cases the desired target concentration of 0.25–2.0 ug/mL was achieved and that there was no need to adapt the dosage.
In summary, F is almost unchanged in pregnant women compared with non-pregnant women. After oral administration, one study showed a lower Cmax in pregnant women. In general, Vd/F of ampicillin is increased in pregnant women compared to non-pregnant women. Furthermore, the reached AUC and CL/F values in pregnant women are lower and higher, respectively, than in non-pregnant women. Moreover, desired target concentrations for ampicillin in pregnant women are achieved with the currently used dosage.
3.1.3 Benzylpenicillin
In total, 4 studies on benzylpenicillin were included in this study (Table 4) [25,26,27,28]. One study investigated second trimester pregnant women, while the other studies included only women during their third trimester of pregnancy. The number of included women in each study varied from 15 to 60. The mean age of the woman ranged from 23.5 to 29.5 years. Mean BMI of the women included in the studies varied from 29.2 to 31.6 kg/m2 and weight varied from 50.9 to 151.7 kg. Mean gestational age ranged from 28.6 to 39.4 weeks. The PK and exposure parameters of benzylpenicillin in pregnant women (where possible, in comparison with non-pregnant women) of the included studies are summarised in Table 4.
No absorption-related PK parameters were reported. Only Johnson et al. reported a Vd in pregnant women during their third trimester, namely 0.27 L/kg [25]. No comparison was made between pregnant and non-pregnant women. The same study also reported CL. The mean total CL in pregnant women during their third trimester was 0.25 L/h/kg [25].
The study of Weeks et al. [28] mentioned a desired target concentration (MIC 0.06 μg/mL) and reported that with their currently used dosing scheme the target concentration will be reached in pregnant women. While Nathan et al. [23] mentioned that in only 40% of the pregnant women, the 0.018 μg/mL target concentration was achieved on Day 7 for congenital syphilis. No adapted evidence-based dosing regimen was provided.
In summary, as limited information is available on the PK changes of benzylpenicillin during pregnancy, no statement can be made on changes in PK of benzylpenicillin in pregnant compared with non-pregnant women. The same holds for the attainment of the target concentration.
3.1.4 Phenoxymethylpenicillin
One study on phenoxymethylpenicillin was included in this study (Table 5) [29], which included second and third trimester pregnant women (n = 12), which were compared to non-pregnant women (n = 6). Table 2 provides the characteristics of the included PK study and the PK-related changes of oral phenoxymethylpenicillin used throughout pregnancy.
When focusing on absorption related parameters (Table 5); the Cmax was 33.9% and 3.5% lower in the second and third trimester, respectively, in pregnant women compared to non-pregnant women. When focusing on Vd; this parameter has not been reported. However, when calculating Vd based on the reported CL and t1/2, the Vd was 55.2% and 14.4% lower in the second and third trimester of pregnancy compared to non-pregnant women. As for the elimination-related parameters, AUC was 52.1% and 39.2% lower and consequently CL was 68% and 47.4% higher in the second and third trimester compared to non-pregnant women.
The authors stated that to reach an adequate target concentration in the second and third trimester of the pregnancy, either the dosing interval must be shorter, namely from 8 to 6 h with a dose of 1 million IU, or dosages should be increased. The authors did not provide an increased evidence-based dosing regimen administered every 8 h.
In summary, Cmax is lower in pregnant and non-pregnant women. In general, Vd of phenoxymethylpenicillin is lower, whereas AUC was lower and consequently CL is higher during pregnancy compared to non-pregnant women.
3.1.5 Piperacillin (With and Without Tazobactam)
In total, 4 studies were included in this study (Table 6) 1 of which investigated piperacillin with tazobactam, and 3 studies without tazobactam. All studies investigated second and third trimester pregnant women or pregnant women during delivery. Bourget et al. [30] and Heikkela et al. [29] compared pregnant women to non-pregnant women, while Voigt et al. [31] only studied PK in pregnant women and compared the results with PK data of non-pregnant women from literature. The number of included women in each study varied from 3 to 12. The mean age of the women ranged from 19 to 37 years. Mean weight of the women included in the studies varied from 60 to 73.5 kg. The mean gestational age ranged from 22–40 weeks. Table 6 provides the characteristics of the included PK study and the PK-related changes of piperacillin (with and without tazobactam) used throughout pregnancy.
When considering distribution-related parameters for piperacillin and tazobactam; Cmax (43.5–49.2% and 47.4%) was lower in second, and third trimester pregnant women, respectively, compared to non-pregnant women [29, 30]. Volume of distribution of piperacillin and tazobactam was 45.5–84.9% and 67.5% higher in second and third trimester pregnant women compared to non-pregnant women. As for the elimination-related PK parameters, AUC (13.9–41.1% and 53.9%) of piperacillin and tazobactam was lower, while CL was 80%–184.8% and 127.1% higher, respectively, in second and third trimester compared to non-pregnant women.
Of the 4 studies, 2 [29, 30] reported a desired target concentration (dependent on the micro-organism, Table 2) and the probability of target attainment with the currently used dosing regimens of piperacillin with or without tazobactam in pregnant women. All 4 studies reported the need of a higher dose to increase the probability of target attainment in pregnant women. However, studies did not report the required evidence-based dosing to obtain target attainment.
In summary, Vd is lower during pregnancy compared to the non-pregnant state resulting in a higher Cmax. Finally, AUC was lower in pregnant compared to non-pregnant women, caused by a higher CL in pregnant women.
4 Discussion
Penicillins belong to the most prescribed drugs for pregnant women to treat infectious diseases. Based on this systematic literature review, we can conclude that current knowledge on the PK, exposure and target attainment of penicillins in pregnant women is relatively scarce. An important finding in our study is that pregnant women during their second and third trimester generally show a higher CL and larger Vd than non-pregnant or postpartum women, resulting in lower concentrations and AUC. Target attainment often appears suboptimal in the pregnant population when using standard doses of different penicillins. For penicillins, it is necessary that the target concentration will be reached as it is known that efficacy is supported by reaching the PK/PD parameter time that the free fraction is above the MIC (fT>MIC) [7, 8]. In case of not reaching the target concentrations, adjustments will be necessary.
Within the limited number of performed PK studies on penicillins during pregnancy, focus is primarily on pregnant women in the second and third trimester. Many studies included women with increased risk for infections, for example, women with premature rupture of membranes or women with other pregnancy-related complications. As pregnant women are admitted to a clinical setting with complications mostly in their second half of pregnancy, the opportunity arises to collect information on PK/PD. Overall, 10/32 articles included information on both Vd and CL. In addition, only a minority of included articles (7/32) provide dosing guidance for clinicians of which 4 studies for amoxicillin, 1 study for ampicillin, as well as for benzylpenicillin, phenoxymethylpenicillin and piperacillin/tazobactam. Another limitation of the included PK studies is the limited heterogenicity of the included patient populations; besides including only second and third trimester pregnant women, comorbidities and co-medication were also excluded from most studies. Furthermore, in most studies only singleton pregnancies were studied. These included homogeneous populations probably influence the quantification and generalisability of the variability within PK parameters in the entire pregnant population.
A limited number of studies have reported PK of penicillins in pregnant women. Even fewer studies have investigated which PD targets are needed as well as the probability of target attainment for the various penicillins (7/32), even when the necessary PK information is available. A logical next step for future research is to study the PK of penicillins in larger heterogenous pregnant populations to better define exposure targets. It has to be noted, that besides investigating the PK, the predefined PK/PD relationships currently used to develop evidence-based dosing regimens also need to be investigated further, as they are mainly based on theoretical concepts and studies in critically ill patients. Thereafter it can be determined which evidence-based doses are needed to attain target concentrations to consequently enlarge target attainment. This is already adequately studied within the group of penicillins for amoxicillin during the second and third trimester of pregnancy (Table 2) using population PK modelling, but needs to be expanded to the other penicillins. Besides using population PK models to develop evidence-based dosing regimens, physiologically based PK approaches can provide a possible contribution as well in the adjustment of dosages for pregnant women (accounting for the foetus) in future research. Furthermore, another limitation of the performed studies is that when focusing on the PK of amoxicillin throughout pregnancy (but likely also generalisable to other penicillins), no distinction can be made between increased GFR or increased renal section as no 24-h urine clearance has been collected in any of the performed PK studies. This is an area that should be investigated in future research.
Finally, our systematic review itself also has some limitations. First, this systematic literature review is limited to PK and target attainment in pregnant women, without including additional PK or safety data on the foetus. Information obtained from cord blood levels from intrapartum women with streptococcus prophylaxis is highly necessary for the preventions of maternal-foetal infections. Approximately 50% of the included PK penicillin studies conducted also reported cord blood levels, which can be extracted and obtained from those studies for future research to investigate maternal-foetal PK of penicillins. Second, although PD parameters (PTA and MIC) are collected, clinical endpoints are not addressed within our systematic literature review. It has to be noted that it could still be possible that potentially under-exposed pregnant women heal as well.
5 Conclusion
This systematic literature review shows that the PK of most penicillins is significantly altered throughout pregnancy. For most penicillins, both Vd and CL are increased. As a consequence, pregnant women have an increased risk for suboptimal target attainment. The larger Vd and higher CL in pregnant women might justify a higher dose or a shorter dose interval of penicillins in this special population to increase target attainment. Studies frequently do not provide dosing advice for pregnant women, not even when the necessary PK information is available. Our study shows gaps in current knowledge and encourages future researchers to provide dosing advice for pregnant women whenever possible.
References
Stojanova J, Arancibia M, Ghimire S, Sandaradura I. Understanding the pharmacokinetics of antibiotics in pregnancy: Is there a role for therapeutic drug monitoring? A narrative review. Therap Drug Monit. 2022;44:1.
Rac H, et al. common bacterial and viral infections: review of management in the pregnant patient. Ann Pharmacother. 2019;53(6):639–51.
Dallmann A, Mian P, Van den Anker J, Allegaert K. Clinical pharmacokinetic studies in pregnant women and the relevance of pharmacometric tools. Curr Pharm Des. 2019;25(5):483–95.
Pariente G, Leibson T, Carls A, Adams-Webber T, Ito S, Koren G. Pregnancy-associated changes in pharmacokinetics: A systematic review. PLOS Med. 2016;1:1.
“PRISMA.” [Online]. Available: https://prisma-statement.org/. Accessed 29 Jun 2022.
Pemberton MN, Oliver RJ, Theaker ED. Miconazole oral gel and drug interactions. Br Dent J. 2004;196(9):529–31.
Fratoni AJ, Nicolau DP, Kuti JL. A guide to therapeutic drug monitoring of β-lactam antibiotics. Pharmacotherapy. 2021;41(2):220–33.
Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: An update. J Antimicrob Chemother. 2005;55(5):601–7.
Andrew MA, et al. Amoxicillin pharmacokinetics in pregnant women: Modeling and simulations of dosage strategies. Clin Pharmacol Ther. 2007;81(4):547–56.
Buckingham M, Welply G, Miller JF, Elstein M. Gastro-intestinal absorption and transplacental transfer of amoxycillin during labour and the influence of metoclopramide. Curr Med Res Opin. 1975;3(6):392–6.
Muller AE, et al. Amoxicillin pharmacokinetics in pregnant women with preterm premature rupture of the membranes. Am J Obstet Gynecol. 2008;198(1):108.e1-108.e6.
Muller AE, et al. The influence of labour on the pharmacokinetics of intravenously administered amoxicillin in pregnant women. Br J Clin Pharmacol. 2008;66(6):866–74.
Muller AE, et al. Pharmacokinetics of amoxicillin in maternal, umbilical cord, and neonatal sera. Antimicrob Agents Chemother. 2009;53(4):1574–80.
Zaręba-Szczudlik J, Romejko-Wolniewicz E, Lewandowski Z, Rózańska H, Czajkowski K. Concentration of amoxicillin in maternal serum, cord blood, amniotic fluid and the placenta after vaginal administration. J Matern Neonatal Med. 2015;28(17):2048–52.
Zarȩba-Szczudlik J, et al. Evaluation of the amoxicillin concentrations in amniotic fluid, placenta, umbilical cord blood and maternal serum two hours after oral administration. Neuroendocrinol Lett. 2017;38(7):502–8.
Zarȩba-Szczudlik J, et al. Evaluation of the amoxicillin concentrations in amniotic fluid, placenta, umbilical cord blood and maternal serum two hours after intravenous administration. Neuroendocrinol Lett. 2017;38(7):502–8.
Assael BM, Como ML, Miraglia M, Pardi G, Sereni F. Ampicillin kinetics in pregnancy. Br J Clin Pharmocol. 1979;1:286–8.
Bloom SL, Cox SM, Bawdon RE, Gilstrap LC. Ampicillin for neonatal group B streptococcal prophylaxis: How rapidly can bactericidal concentrations be achieved? Am J Obstet Gynecol. 1996;175(4):974–6.
Bray RE, Boe RW, Johnson WL. Transfer of ampicillin into fetus and amniotic fluid from maternal plasma in late pregnancy. Am J Obstet Gynecol. 1966;96(7):938–42.
Chamberlain A, White S, Bawdon R, Thomas S, Larsen B. Pharmacokinetics of ampicillin and sulbactam in pregnancy. Am J Obstet Gynecol. 1993;168(2):667–73.
Colombo DF, Lew JL, Pedersen CA, Johnson JR, Fan-Havard P. Optimal timing of ampicillin administration to pregnant women for establishing bactericidal levels in the prophylaxis of Group B Streptococcus. Am J Obstet Gynecol. 2006;194(2):466–70.
Philipson A. Pharmacokinetics of ampicillin during pregnancy. J Infect Dis. 1977;136(3):370–6.
Creatsas, G, Pavlatos M, Lolis D, Kaskarelis D. Ampicillin and gentamicin in the treatment of fetal intrauterine infections. 1980;8.
Morales WJ, Lim DV, Walsh AF. Prevention of neonatal group B streptococcal sepsis by the use of a rapid screening test and selective intrapartum chemoprophylaxis. Am J Obstet Gynecol. 1986;155(5):979–83.
Johnson JR, Colombo DF, Gardner D, Cho E, Fan-Havard P, Shellhaas CS. Optimal dosing of penicillin G in the third trimester of pregnancy for prophylaxis against group B Streptococcus. Am J Obstet Gynecol. 2001;185(4):850–3.
Nathan L, Bawdon RE, Sidawi JE, Stettler RW, McIntire DM, Wendel GD. Penicillin levels following the administration of benzathine penicillin G in pregnancy. Obstet Gynecol. 1993;82(3):338–42.
Scasso S, Laufer J, Rodriguez G, Alonso JG, Sosa CG. Vaginal group B streptococcus status during intrapartum antibiotic prophylaxis. Int J Gynecol Obstet. 2015;129(1):9–12.
Weeks J, et al. Persistence of benzathine penicillin in pregnant group b strep carriers. Acta Diabetol Lat. 1997;176(1):240–3.
Heikkiiä A, Erkkola R. Pharmacokinetics of piperacillin during pregnancy. J Antimicrob Chemother. 1991;28(3):419–23.
Bourget P, Sertin A, Lesne-Hulin A, Fernandez H, Ville Y, Van Peborgh P. Influence of pregnancy on the pharmacokinetic behaviour and the transplacental transfer of the piperacillin-tazobactam combination. Eur J Obstet Gynecol Reprod Biol. 1998;76(1):21–7.
Voigt R, Peiker G. Late pregnancy. 1985; 424:417–424.
Gilstrap III LC, Bawdon RE. Antibiotic concentration in maternal blood, cord blood, and placental membranes in chorioamnionitis. Obstet. Gynecol. 1988.
Kubacka RT, Johnstone HE, Tan HSI, Reeme PD, Myre SA. Intravenous ampicillin pharmacokinetics in the third trimester of pregnancy. Ther Drug Monit. 1983;5(1):55–60.
Nöschel HH et al. Research on pharmacokinetics during pregnancy. 1980.
Philipson A. Plasma levels of ampicillin in pregnant women following administration of ampicillin and pivampicillin. Am J Obstet Gynecol. 1978;130(6):674–83.
Heikkiiä A, Erkkola RU. The need for adjustment of dosage regimen of penicillin v during pregnancy. Obst Gynecol. 1993;1:1.
Brown CEL, Christmas JT, Bawdon RE. Placental transfer of cefazolin and piperacillin in pregnancies remote from term complicated by Rh isoimmunization. Am J Obstet Gynecol. 1990;163(3):938–43.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors. Competing interests Melanie R. Hesse, Jelmer R. Prins, Marjolijn N. Lub-de Hooge, Rik L.J. Winter, Jos G.W. Kosterink, Daniel J. Touw and Paola Mian declare that they have no financial nor non-financial interests that are directly or indirectly related to the work submitted for publication.
Ethics approval
Not applicable
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability statement
Not applicable
Code availability
Not applicable
Authors’ contributions
MRH contributed to the literature search, data-extraction, data-analysis and writing the initial draft of the manuscript. JRP, MNLDH, HLJW, JGWK contributed to study design and DJT contributed to conceptualization of the study, data-analysis and reviewing the manuscript. PM contributed to the conceptualisation of the study, literature search, data extraction, data analysis and writing and reviewing the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hesse, M.R., Prins, J.R., Hooge, M.N.Ld. et al. Pharmacokinetics and Target Attainment of Antimicrobial Drugs Throughout Pregnancy: Part I—Penicillins. Clin Pharmacokinet 62, 221–247 (2023). https://doi.org/10.1007/s40262-023-01211-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01211-z