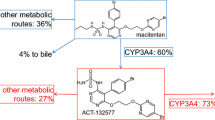
Abstract
Background
Macitentan and its active metabolite, aprocitentan, are non-peptide, potent, dual endothelin receptor antagonists. Macitentan is approved for the treatment of pulmonary arterial hypertension in adults, at a dose of 10 mg/day.
Objective
The objective of this study was to develop a comprehensive population model to describe the pharmacokinetics of macitentan and aprocitentan in healthy adults and adult subjects with pulmonary arterial hypertension.
Methods
Pharmacokinetic data of 452 subjects in nine studies, after single and repeated doses (dose range 0.2–600 mg), were pooled for a non-linear mixed-effects analysis and the assessment of covariates, i.e., body weight, age, sex, race, renal and hepatic impairment, health status (healthy volunteers vs patients with pulmonary arterial hypertension), and formulation (capsules vs tablets) on pharmacokinetic parameters.
Results
The final model was an open one-compartment disposition model, with linear elimination for macitentan and linear formation and elimination for aprocitentan. A semi-mechanistic absorption model described the dose dependency and multiple peaks observed for macitentan. For a female patient with pulmonary arterial hypertension after oral administration at 10 mg, macitentan reached a maximum concentration after 9 h and, following daily dosing, reached steady state after 3 days with a twofold accumulation factor. The apparent volume of distribution was 34 L and clearance was 1.39 L/h. Aprocitentan reached maximum concentration after 51 h and steady state after 9 days, with a 12.5-fold accumulation factor. Body weight, sex, race, renal impairment, health status, and formulation were statistically significant covariates on pharmacokinetic parameters.
Conclusions
The comprehensive population pharmacokinetic model adequately described the pharmacokinetics of macitentan and aprocitentan across different dose concentrations, regimens, and formulations. Several covariates significantly influenced the pharmacokinetics of macitentan and aprocitentan, but none was considered clinically relevant.




Similar content being viewed by others
References
Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351(14):1425–36.
Sidharta PN, Treiber A, Dingemanse J. Clinical pharmacokinetics and pharmacodynamics of the endothelin receptor antagonist macitentan. Clin Pharmacokinet. 2015;54(5):457–71.
Dingemanse J, Sidharta PN, Maddrey WC, Rubin LJ, Mickail H. Efficacy, safety and clinical pharmacology of macitentan in comparison to other endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Expert Opin Drug Saf. 2014;13(3):391–405.
US Food and Drug Administration. Opsumit® (macitentan) prescribing information. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204410s017lbl.pdf. Accessed 26 Nov 2020.
EMC. Summary of product characteristics: Opsumit (macitentan). 2020. https://www.medicines.org.uk/emc/product/5223/smpc. Accessed 26 Nov 2020.
Atsmon J, Dingemanse J, Shaikevich D, Volokhov I, Sidharta PN. Investigation of the effects of ketoconazole on the pharmacokinetics of macitentan, a novel dual endothelin receptor antagonist, in healthy subjects. Clin Pharmacokinet. 2013;52(8):685–92.
Krause A, Zisowsky J, Dingemanse J. Modeling of pharmacokinetics, efficacy, and hemodynamic effects of macitentan in patients with pulmonary arterial hypertension. Pulm Pharmacol Ther. 2018;49:140–6.
Sidharta PN, van Giersbergen PL, Halabi A, Dingemanse J. Macitentan: entry-into-humans study with a new endothelin receptor antagonist. Eur J Clin Pharmacol. 2011;67(10):977–84.
Sidharta PN, van Giersbergen PL, Dingemanse J. Safety, tolerability, pharmacokinetics, and pharmacodynamics of macitentan, an endothelin receptor antagonist, in an ascending multiple-dose study in healthy subjects. J Clin Pharmacol. 2013;53(11):1131–8.
US Food and Drug Administration. Clinical pharmacology and biopharmaceutics review. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204410Orig1s000ClinPharmR.pdf. Accessed 26 Nov 2020.
Bruderer S, Marjason J, Sidharta PN, Dingemanse J. Pharmacokinetics of macitentan in Caucasian and Japanese subjects: the influence of ethnicity and sex. Pharmacology. 2013;91(5–6):331–8.
Ahn LY, Kim SE, Yi S, et al. Pharmacokinetic–pharmacodynamic relationships of macitentan, a new endothelin receptor antagonist, after multiple dosing in healthy Korean subjects. Am J Cardiovasc Drugs. 2014;14(5):377–85.
Sidharta PN, Lindegger N, Ulč I, Dingemanse J. Pharmacokinetics of the novel dual endothelin receptor antagonist macitentan in subjects with hepatic or renal impairment. J Clin Pharmacol. 2014;54(3):291–300.
Kummer O, Haschke M, Hammann F, et al. Comparison of the dissolution and pharmacokinetic profiles of two galenical formulations of the endothelin receptor antagonist macitentan. Eur J Pharm Sci. 2009;38(4):384–8.
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ, editors. NONMEM 7.3.0 users guide (1989–2014). Ellicott City: Icon Development Solutions.
R Archive Network. http://cran.r-project.org. Accessed 26 Nov 2020.
de Kanter R, Sidharta PN, Delahaye S, et al. Physiologically-based pharmacokinetic modeling of macitentan: prediction of drug–drug interactions. Clin Pharmacokinet. 2016;55(3):369–80.
US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. 2003. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072123.pdf. Accessed 26 Nov 2020.
US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired renal function: study design, data analysis, and impact on dosing and labeling. 2010. https://www.fda.gov/downloads/drugs/guidances/ucm204959.pdf. Accessed 26 Nov 2020.
Xu XS, Yuan M, Zhu H, et al. Full covariate modelling approach in population pharmacokinetics: understanding the underlying hypothesis tests and implications of multiplicity. Br J Clin Pharmacol. 2018;84(7):1525–34.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Bauer RJ. NONMEM tutorial part II: estimation methods and advanced examples. CPT Pharmacomet Syst Pharmacol. 2019;8(8):538–56.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM. J Pharmacokinet Pharmacodyn. 2008;35(4):401–21.
Volz AK, Dingemanse J, Krause A, Lehr T. Target-mediated population pharmacokinetic modeling of endothelin receptor antagonists. Pharm Res. 2020;37(1):1–12.
Bruderer S, Hopfgartner G, Seiberling M, et al. Absorption, distribution, metabolism, and excretion of macitentan, a dual endothelin receptor antagonist, in humans. Xenobiotica. 2012;42(9):901–10.
Sidharta PN, Melchior M, Kankam MK, Dingemanse J. Single- and multiple-dose tolerability, safety, pharmacokinetics, and pharmacodynamics of the dual endothelin receptor antagonist aprocitentan in healthy adult and elderly subjects. Drug Des Dev Ther. 2019;13:949–64.
Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ contributions
RB, AGD, DC, JJPR, PM, and IP contributed to the design of the work, the acquisition of the data, the analysis or interpretation of the data, and the preparation of the manuscript. All authors have approved the submitted version and are accountable for it.
Conflicts of interest
Anne-Gaëlle Dosne, Dénes Csonka, Juan José Pérez-Ruixo, and Italo Poggesi were employees and shareholders of Janssen Pharmaceutical Companies at the time this analysis was conducted.
Funding
The clinical studies were supported by research funding from Actelion (currently, a Janssen pharmaceutical company).
Ethics approval
The studies were conducted in accordance with principles for human experimentation as defined in the Declaration of Helsinki and were approved by the human investigational review board of the study center and by the competent authority of the country.
Consent to participate
Informed consent was obtained from each subject before enrollment in the studies, after being advised of the potential risks and benefits, as well as the investigational nature of the studies.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
The NONMEM script of the final model is available as ESM.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bartolucci, R., Dosne, AG., Csonka, D. et al. A Population Pharmacokinetic Model of Macitentan and Its Active Metabolite Aprocitentan in Healthy Volunteers and Patients with Pulmonary Arterial Hypertension. Clin Pharmacokinet 60, 1605–1619 (2021). https://doi.org/10.1007/s40262-021-01049-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01049-3