Abstract
Introduction
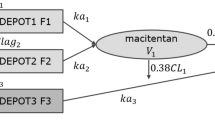
Macitentan is a novel dual endothelin receptor antagonist for the treatment of pulmonary arterial hypertension (PAH). It is metabolized by cytochrome P450 (CYP) enzymes, mainly CYP3A4, to its active metabolite ACT-132577.
Methods
A physiological-based pharmacokinetic (PBPK) model was developed by combining observations from clinical studies and physicochemical parameters as well as absorption, distribution, metabolism and excretion parameters determined in vitro.
Results
The model predicted the observed pharmacokinetics of macitentan and its active metabolite ACT-132577 after single and multiple dosing. It performed well in recovering the observed effect of the CYP3A4 inhibitors ketoconazole and cyclosporine, and the CYP3A4 inducer rifampicin, as well as in predicting interactions with S-warfarin and sildenafil. The model was robust enough to allow prospective predictions of macitentan–drug combinations not studied, including an alternative dosing regimen of ketoconazole and nine other CYP3A4-interacting drugs. Among these were the HIV drugs ritonavir and saquinavir, which were included because HIV infection is a known risk factor for the development of PAH.
Conclusion
This example of the application of PBPK modeling to predict drug–drug interactions was used to support the labeling of macitentan (Opsumit).






Similar content being viewed by others
References
Gatfield J, Mueller Grandjean C, Sasse T, Clozel M, Nayler O. Slow receptor dissociation kinetics differentiate macitentan from other endothelin receptor antagonists in pulmonary arterial smooth muscle cells. PloS One. 2012;7:e47662.
Iglarz M, Binkert C, Morrison K, Fischli W, Gatfield J, Treiber A, et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther. 2008;327:736–45.
Treiber A, Aanismaa P, de Kanter R, Delahaye S, Treher M, Hess P, et al. Macitentan does not interfere with hepatic bile salt transport. J Pharmacol Exp Ther. 2014;350:130–43.
Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62:D60–72.
Cicalini S, Chinello P, Petrosillo N. HIV infection and pulmonary arterial hypertension. Expert Rev Respir Med. 2011;5:257–66.
Jones H, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacomet Syst Pharmacol. 2013;2:1–12.
Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24:53–75.
Jones HM, Gardner IB, Watson KJ. Modelling and PBPK simulation in drug discovery. AAPS J. 2009;11:155–66.
Edginton AN, Theil FP, Schmitt W, Willmann S. Whole body physiologically-based pharmacokinetic models: their use in clinical drug development. Expert Opin Drug Metab Toxicol. 2008;4:1143–52.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Ann Rev Pharmacol Toxicol. 2011;51:45–73.
Huang SM, Rowland M. The role of physiologically based pharmacokinetic modeling in regulatory review. Clin Pharmacol Ther. 2012;91:542–9.
Sinha V, Zhao P, Huang SM, Zineh I. Physiologically based pharmacokinetic modeling: from regulatory science to regulatory policy. Clin Pharmacol Ther. 2014;95:478–80.
Opsumit (macitentan): summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002697/WC500160899.pdf. Accessed 21 July 2015.
Atsmon J, Dingemanse J, Shaikevich D, Volokhov I, Sidharta PN. Investigation of the effects of ketoconazole on the pharmacokinetics of macitentan, a novel dual endothelin receptor antagonist, in healthy subjects. Clin Pharmacokinet. 2013;52:685–92.
Bruderer S, Aanismaa P, Homery MC, Hausler S, Landskroner K, Sidharta PN, et al. Effect of cyclosporine and rifampin on the pharmacokinetics of macitentan, a tissue-targeting dual endothelin receptor antagonist. AAPS J. 2012;14:68–78.
Sidharta PN, van Giersbergen PL, Wolzt M, Dingemanse J. Investigation of mutual pharmacokinetic interactions between macitentan, a novel endothelin receptor antagonist, and sildenafil in healthy subjects. Br J Clin Pharmacol. 2014;78:1035–42.
Sidharta PN, Dietrich H, Dingemanse J. Investigation of the effect of macitentan on the pharmacokinetics and pharmacodynamics of warfarin in healthy male subjects. Clin Drug Investig. 2014;34:545–52.
van Giersbergen PL, Gnerre C, Treiber A, Dingemanse J, Meyer UA. Bosentan, a dual endothelin receptor antagonist, activates the pregnane X nuclear receptor. Eur J Pharmacol. 2002;450:115–21.
Proctor N, Tucker G, Rostami-Hodjegan A. Predicting drug clearance from recombinantly expressed CYPs: intersystem extrapolation factors. Xenobiotica. 2004;34:151–78.
Bruderer S, Hopfgartner G, Seiberling M, Wank J, Sidharta PN, Treiber A, et al. Absorption, distribution, metabolism, and excretion of macitentan, a dual endothelin receptor antagonist, in humans. Xenobiotica. 2012;42:901–10.
Center for Drug Evaluation and Research. Application number 204410Orig1s000. Clinical pharmacology and biopharmaceutics review(s). 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204410Orig1s000ClinPharmR.pdf. Accessed 21 July 2015.
Yang J, Jamei M, Yeo KR, Tucker GT, Rostami-Hodjegan A. Prediction of intestinal first-pass drug metabolism. Curr Drug Metab. 2007;8:676–84.
Pang KS, Chow EC. Commentary: theoretical predictions of flow effects on intestinal and systemic availability in physiologically based pharmacokinetic intestine models: the traditional model, segregated flow model, and QGut model. Drug Metab Dispos. 2012;40:1869–77.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95:1238–57.
Rowland M, Tozer T. Metabolite kinetics, Chap 21. In: Clinical pharmacokinetics: concepts and applications. 3rd ed. Media, PA: Lippincott Williams & Wilkins; 1994. p. 372.
Sidharta PN, van Giersbergen PL, Dingemanse J. Safety, tolerability, pharmacokinetics, and pharmacodynamics of macitentan, an endothelin receptor antagonist, in an ascending multiple-dose study in healthy subjects. J Clin Pharmacol. 2013;53:1131–8.
Wagner C, Pan Y, Hsu V, Grillo JA, Zhang L, Reynolds KS, et al. Predicting the effect of cytochrome P450 inhibitors on substrate drugs: analysis of physiologically based pharmacokinetic modeling submissions to the US Food and Drug Administration. Clin Pharmacokinet. 2015;54:117–27.
Darwich AS, Neuhoff S, Jamei M, Rostami-Hodjegan A. Interplay of metabolism and transport in determining oral drug absorption and gut wall metabolism: a simulation assessment using the “Advanced Dissolution, Absorption, Metabolism (ADAM)” model. Curr Drug Metab. 2010;11:716–29.
Zisowsky J, Sidharta P, Krause A, Dingemanse J. Pharmacokinetic/pharmacodynamic analyses in SERAPHIN, a randomized, controlled study of macitentan in patients with pulmonary arterial hypertension. Clin Pharmacol Drug Dev. 2013;2:S29.
Zientek MA, Youdim K. Reaction phenotyping: advances in the experimental strategies used to characterize the contribution of drug-metabolizing enzymes. Drug Metab Dispos. 2015;43:163–81.
Tsamandouras N, Rostami-Hodjegan A, Aarons L. Combining the “bottom-up” and “top-down” approaches in pharmacokinetic modelling: fitting PBPK models to observed clinical data. Br J Clin Pharmacol. 2013;79:48–55.
Zhao P, Ragueneau-Majlessi I, Zhang L, Strong JM, Reynolds KS, Levy RH, et al. Quantitative evaluation of pharmacokinetic inhibition of CYP3A substrates by ketoconazole: a simulation study. J Clin Pharmacol. 2009;49:351–9.
Opsumit (macitentan): US prescribing information. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204410s003lbl.pdf. Accessed 21 July 2015.
Australian Public Assessment Report for macitentan. 2014. http://www.tga.gov.au/file/5941/download. Accessed 21 July 2015.
Baxter JG, Brass C, Schentag JJ, Slaughter RL. Pharmacokinetics of ketoconazole administered intravenously to dogs and orally as tablet and solution to humans and dogs. J Pharm Sci. 1986;75:443–7.
Kummer O, Haschke M, Hammann F, Bodmer M, Bruderer S, Regnault Y, et al. Comparison of the dissolution and pharmacokinetic profiles of two galenical formulations of the endothelin receptor antagonist macitentan. Eur J Pharm Sci. 2009;38:384–8.
Bruderer S, Marjason J, Sidharta PN, Dingemanse J. Pharmacokinetics of macitentan in caucasian and Japanese subjects: the influence of ethnicity and sex. Pharmacology. 2013;91:331–8.
Acknowledgments
The authors would like to thank Fabienne Drouet, Julia Khobzaoui and Swen Seeland for their contributions to the in vitro studies, and Makda Fisseha for help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Actelion Pharmaceuticals Ltd.
Conflicts of interest
Ruben de Kanter, Patricia N. Sidharta, Stéphane Delahaye, Carmela Gnerre, Jerome Segrestaa, Stephan Buchmann, Christopher Kohl and Alexander Treiber are employees of Actelion Pharmaceuticals Ltd and may receive stock or stock options as part of their compensation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Kanter, R., Sidharta, P.N., Delahaye, S. et al. Physiologically-Based Pharmacokinetic Modeling of Macitentan: Prediction of Drug–Drug Interactions. Clin Pharmacokinet 55, 369–380 (2016). https://doi.org/10.1007/s40262-015-0322-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0322-y