Abstract
Introduction
NNC0109-0012, a novel human monoclonal antibody that binds to and neutralizes the activity of interleukin–20, was investigated as a potential treatment for inflammatory diseases. Pharmacokinetic (PK) modeling was performed using data from four completed clinical phase 1/2 trials to better understand the clinical PK of NNC0109-0012.
Methods
The populations included were patients with rheumatoid arthritis (RA), chronic plaque psoriasis, and healthy volunteers. NNC0109-0012 was administered subcutaneously at various dose levels (0.01–3 mg/kg) as single dose, once weekly, or multiple doses every second week for up to 12 doses. Noncompartmental methods were used to describe the PK parameters. Population PK was analyzed using nonlinear mixed-effects modeling, with body weight as the main covariate and gender, age, and population as additional covariates.
Results
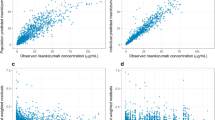
Across studies (N = 116), mean age and body weight ranged from 38 to 58 years and 72 to 96 kg, respectively. NNC0109-0012 displays linear PK. Time to maximum plasma concentration occurred at approximately 1 week, and the terminal half-life was approximately 3 weeks. Clearance and volume of distribution increased proportionally to body weight. No difference in clearance or volume of distribution was observed between gender or different age groups; however, clearance was slightly lower in healthy volunteers than in patients with RA.
Conclusion
The PK profile of NNC0109-0012 is similar to other monoclonal antibodies directed against soluble targets.


Similar content being viewed by others
References
Blumberg H, Conklin D, Xu WF, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104(1):9–19.
Wang F, Lee E, Lowes MA, et al. Prominent production of IL-20 by CD68+/CD11c+ myeloid-derived cells in psoriasis: gene regulation and cellular effects. J Invest Dermatol. 2006;126(7):1590–9.
Wolk K, Kunz S, Asadullah K, et al. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168(11):5397–402.
Dumoutier L, Leemans C, Lejeune D, et al. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167(7):3545–9.
Logsdon NJ, Deshpande A, Harris BD, et al. Structural basis for receptor sharing and activation by interleukin-20 receptor-2 (IL-20R2) binding cytokines. Proc Natl Acad Sci. 2012;109(31):12704–9.
Hsu YH, Li HH, Hsieh MY, et al. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 2006;54(9):2722–33.
Hsu YH, Chang MS. Interleukin-20 antibody is a potential therapeutic agent for experimental arthritis. Arthritis Rheum. 2010;62(11):3311–21.
Kragstrup TW, Otkjaer K, Holm C, et al. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine. 2008;41(1):16–23.
Rømer J, Jackerott M, Mandelbaum J, et al. Expression of interleukin-20 and its receptor chains IL-20R1, IL-20R2 and IL-22R in synovium from patients with rheumatoid arthritis. Ann Rheum Dis. 2012;71(suppl 3):646.
Pass J, Clausen JT, Worsaae A, et al. Characterization of a human monoclonal antibody that neutralizes interleukin-20. Ann Rheum Dis. 2012;71(suppl 3):642.
Šenolt L, Göthberg M, Valencia X, et al. Efficacy and safety of NNC0109-0012 (anti-IL-20 mAb) in patients with rheumatoid arthritis: results from a phase 2a trial. Ann Rheum Dis. 2012;71(suppl 3):152.
Al Kotbi N, Jensen L, Graff LB. FRI0196 NNC0109-0012 (anti-IL-20 MAB), well tolerated in healthy subjects and patients with rheumatoid arthritis. Ann Rheum Dis. 2013;71(suppl 3):379.
Leszczynski P, Eshof M, Stegmann H, et al. FRI0197 NNC0109-0012 (anti-IL-20 mAb), well tolerated in patients with rheumatoid arthritis. Ann Rheum Dis. 2012;71(suppl 3):379–80.
US Department of Health and Human Services Food and Drug Administration. Guidance for industry: bioanalytical method validation. 2001 [cited 2014 February 4]. Available from: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf.
DeSilva B, Smith W, Weiner R, et al. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res. 2003;20(11):1885–900.
Viswanathan CT, Bansal S, Booth B, et al. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res. 2007;24(10):1962–73.
Mascelli MA, Zhou H, Sweet R, et al. Molecular, biologic, and pharmacokinetic properties of monoclonal antibodies: impact of these parameters on early clinical development. J Clin Pharmacol. 2007;47(5):553–65.
Aydogdu E, Pamuk ON, Donmez S, et al. Decreased interleukin-20 level in patients with systemic sclerosis: are they related with angiogenesis? Clin Rheumatol. 2013;32(11):1599–603.
Michalak-Stoma A, Bartosinska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35(6):625–31.
van Dooren FH, Duijvis NW, te Velde AA. Analysis of cytokines and chemokines produced by whole blood, peripheral mononuclear and polymorphonuclear cells. J Immunol Methods. 2013;396(1–2):128–33.
Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14(2):296–302.
Thway TM, Magana I, Bautista A, et al. Impact of anti-drug antibodies in preclinical pharmacokinetic assessment. AAPS J. 2013;15(3):856–63.
Acknowledgments
This study and the associated article processing charges were supported by Novo Nordisk A/S, Denmark. Under the direction of the authors, editorial assistance was provided by Jane Phillips, PhD, and Maryann Travaglini, PharmD, of Complete Healthcare Communications, Inc. (Chadds Ford, PA, USA), and was funded by Novo Nordisk A/S. The authors had full control of the data reported in this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
Mia Sandberg Lundblad is an employee of Novo Nordisk and holds stock options in Novo Nordisk. Rune Viig Overgaard is an employee of Novo Nordisk and holds stock options in Novo Nordisk. Marie Göthberg is an employee of Novo Nordisk and holds stock options in Novo Nordisk. Marianne Scheel Fjording is an employee of Novo Nordisk and holds stock options in Novo Nordisk. Estelle Watson is an employee of Novo Nordisk and holds stock options in Novo Nordisk.
Compliance with ethics guidelines
The clinical trials described in this manuscript were approved by the national health authorities and the local ethics committee, as applicable, and were performed in accordance with the ethical standards in the 1964 Declaration of Helsinki and its later amendments. All clinical trial participants gave their informed consent before inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lundblad, M.S., Overgaard, R.V., Göthberg, M. et al. Clinical Pharmacokinetics of the Anti-Interleukin-20 Monoclonal Antibody NNC0109-0012 in Healthy Volunteers and Patients with Psoriasis or Rheumatoid Arthritis. Adv Ther 32, 228–238 (2015). https://doi.org/10.1007/s12325-015-0191-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0191-7