Abstract
Background and Objectives
Veliparib is an orally active potent poly(ADP-ribose) polymerase (PARP) inhibitor currently in phase III clinical trials in solid tumors. This phase I study evaluated the pharmacokinetics and mass balance of veliparib administered alone and in combination with temozolomide, and assessed any potential pharmacokinetic drug–drug interaction between veliparib and temozolomide.
Methods
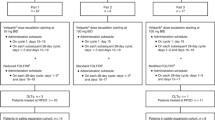
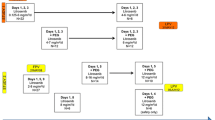
This was an open-label, dose-escalation study of veliparib in combination with temozolomide in 42 subjects with nonhematologic malignancies. Veliparib was administered orally at doses ranging from 10 to 80 mg twice daily on days 1–7, and temozolomide was administered orally at 150–200 mg/m2 once daily on days 1–5 of each 28-day cycle. The pharmacokinetics of veliparib, its M8 metabolite, and temozolomide, as well as urinary excretion of unchanged veliparib and its M8 metabolite, were determined.
Results
Mean veliparib maximum observed plasma concentration (C max) and area under the plasma concentration–time curve for the first 6 h postdose (AUC6) values increased dose proportionally in the veliparib 10–80 mg twice-daily dose range. The urinary recovery of veliparib dose as the unchanged parent compound alone and together with the M8 metabolite was 73 ± 18 and 90 ± 22%, respectively, over a 12-h dosing interval on day 6 of Cycle 1. Veliparib and temozolomide pharmacokinetic exposures were not affected when administered together.
Conclusions
Veliparib is a Biopharmaceutical Classification System (BCS) Class 1 compound, with no less than 90% of the dose absorbed and an oral bioavailability of at least 73%. Veliparib is primarily eliminated by renal excretion. Veliparib exhibited linear pharmacokinetics in the 10–80 mg twice-daily dose range. No pharmacokinetic interaction was observed when veliparib and temozolomide were administered together.
Clinical Trial Registration Number: NCT00526617.



Similar content being viewed by others
References
de Murcia G, Ménissier-de Murcia J, Schreiber V. Poly(ADP-ribose) polymerase: molecular biological aspects. BioEssays. 1991;13:455–62.
D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342(Pt 2):249–68.
Schreiber V, Amé J-C, Dollé P, Schultz I, Rinaldi B, Fraulob V, et al. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277:23028–36.
DePinho RA, Polyak K. Cancer chromosomes in crisis. Nat Genet. 2004;36:932–4.
Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012;12:587–98.
Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–37.
Murai J, Huang SN, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–99.
Hopkins TA, Shi Y, Rodriguez LE, Solomon LR, Donawho CK, DiGiammarino EL, et al. Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol Cancer Res. 2015;13:1465–77.
Owonikoko TK, Zhang G, Deng X, Rossi MR, Switchenko JM, Doho GH, et al. Poly (ADP) ribose polymerase enzyme inhibitor, veliparib, potentiates chemotherapy and radiation in vitro and in vivo in small cell lung cancer. Cancer Med. 2014;3:1579–94.
Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med. 1995;14:1149–61.
Piantadosi S, Fisher JD, Grossman S. Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol. 1998;41:429–36.
Mostafa NM, Chiu Y-L, Rosen LS, Bessudo A, Kovacs X, Giranda VL. A phase 1 study to evaluate effect of food on veliparib pharmacokinetics and relative bioavailability in subjects with solid tumors. Cancer Chemother Pharmacol. 2014;74:583–91.
Temodar [package insert]. Kenilworth, NJ: Schering Corporation; 2005.
Parise RA, Shawaqfeh M, Egorin MJ, Beumer JH. Liquid chromatography-mass spectrometric assay for the quantitation in human plasma of ABT-888, an orally available, small molecule inhibitor of poly(ADP-ribose) polymerase. J Chromatogr B. 2008;872:141–7.
Penning TD, Zhu G-D, Gandhi VB, Gong J, Liu X, Shi Y, et al. Discovery of the poly(ADP-ribose) polymerase (PARP) inhibitor 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide (ABT-888) for the treatment of cancer. J Med Chem. 2009;52:514–23.
Li X, Delzer J, Voorman R, de Morais SM, Lao Y. Disposition and drug-drug interaction potential of veliparib (ABT-888), a novel and potent inhibitor of poly(ADP-ribose) polymerase. Drug Metab Dispos. 2011;39:1161–9.
Kikuchi R, Lao Y, Bow DAJ, Chiou WJ, Andracki ME, Carr RA, et al. Prediction of clinical drug-drug interactions of veliparib (ABT-888) with human renal transporters (OAT1, OAT3, OCT2, MATE1, and MATE2K). J Pharm Sci. 2013;102:4426–32.
Li J, Kim S, Sha X, Wiegand R, Wu J, LoRusso P. Complex disease-, gene-, and drug-drug interactions: impacts of renal function, CYP2D6 phenotype, and OCT2 activity on veliparib pharmacokinetics. Clin Cancer Res. 2014;20:3931–44.
Baker SD, Wirth M, Statkevich P, Reidenberg P, Alton K, Sartorius SE, et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res. 1999;5:309–17.
LoRusso PM, Li J, Burger A, Heilbrun LK, Sausville EA, Boerner SA, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in combination with irinotecan in patients with advanced solid tumors. Clin Cancer Res. 2016;22:3227–37.
Mizugaki H, Yamamoto N, Nokihara H, Fujiwara Y, Horinouchi H, Kanda S, et al. A phase 1 study evaluating the pharmacokinetics and preliminary efficacy of veliparib (ABT-888) in combination with carboplatin/paclitaxel in Japanese subjects with non-small cell lung cancer (NSCLC). Cancer Chemother Pharmacol. 2015;76:1063–72.
Lynparza(R) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2014.
Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19:5003–15.
Zhang Z-Y, Wang X, Lu S, Wang J, Agarwal S, Martell R, et al. Biotransformation and disposition of niraparib, an investigational, selective human PARP-1 and PARP-2 antagonist, in vitro. Drug Metab Rev. 2016;48(52):P53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
AbbVie Inc. provided financial support for the study and participated in the design, study conduct, analysis and interpretation of data, as well as the writing, review and approval of the manuscript.
Conflict of interest
Silpa Nuthalapati, Wijith Munasinghe, Vincent Giranda, and Hao Xiong are employees of AbbVie Inc. and may hold AbbVie stock or stock options.
Rights and permissions
About this article
Cite this article
Nuthalapati, S., Munasinghe, W., Giranda, V. et al. Clinical Pharmacokinetics and Mass Balance of Veliparib in Combination with Temozolomide in Subjects with Nonhematologic Malignancies. Clin Pharmacokinet 57, 51–58 (2018). https://doi.org/10.1007/s40262-017-0547-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0547-z