Abstract
Background and Objectives
Electronic cigarettes (e-cigarettes) are a recent technology that has gained rapid acceptance. Still, little is known about them in terms of safety and effectiveness. A basic question is how effectively they deliver nicotine; however, the literature is surprisingly unclear on this point. Here, a population pharmacokinetic model was developed for nicotine and its major metabolite cotinine with the aim to provide a reliable framework for the simulation of nicotine and cotinine concentrations over time, based solely on inhalation airflow recordings and individual covariates [i.e., weight and breath carbon monoxide (CO) levels].
Methods
This study included ten adults self-identified as heavy smokers (at least one pack of cigarettes per day). Plasma nicotine and cotinine concentrations were measured at regular 10-min intervals for 90 min while human subjects inhaled nicotine vapor from a modified e-cigarette. Airflow measurements were recorded every 200 ms throughout the session. A population pharmacokinetic model for nicotine and cotinine was developed based on previously published pharmacokinetic parameters and the airflow recordings. All of the analyses were performed with the non-linear mixed-effect modeling software NONMEM® version 7.2.
Results
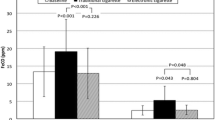
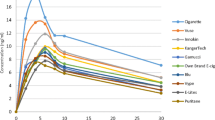
The results show that e-cigarettes deliver nicotine effectively, although the pharmacokinetic profiles are lower than those achieved with regular cigarettes. Our pharmacokinetic model effectively predicts plasma nicotine and cotinine concentrations from the inhalation volume, and initial breath CO.
Conclusion
E-cigarettes are effective at delivering nicotine. This new pharmacokinetic model of e-cigarette usage might be used for pharmacodynamic analysis where the pharmacokinetic profiles are not available.







Similar content being viewed by others
References
Yamazaki H, Horiuchi K, Takano R, Nagano T, Shimizu M, Kitajima M, et al. Human blood concentrations of cotinine, a biomonitoring marker for tobacco smoke, extrapolated from nicotine metabolism in rats and humans and physiologically based pharmacokinetic modeling. Int J Environ Res Public Health. 2010;7:3406–21.
Teeguarden JG, Housand CJ, Smith JN, Hinderliter PM, Gunawan R, Timchalk CA. A multi-route model of nicotine–cotinine pharmacokinetics, pharmacodynamics and brain nicotinic acetylcholine receptor binding in humans. Regul Toxicol Pharmacol. 2013;65:12–28.
Robinson DE, Balter NJ, Schwartz SL. A physiologically based pharmacokinetic model for nicotine and cotinine in man. J Pharmacokinet Biopharm. 1992;20:591–609.
Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–28.
Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8:e66317.
Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23(Suppl 2):ii11–7.
Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–86.
Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl). 2014;231:401–7.
Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–70.
Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem Toxicol. 2012;50:3600–3.
Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control. 2014;23(Suppl 2):ii30–5.
Hajek P, Goniewicz ML, Phillips A, Myers SK, West O, McRobbie H. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. Epub 2014 Aug 13.
Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–53.
Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19:87–8.
Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117:758–63.
Hukkanen J, Jacob P III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115.
Armitage AK, Dollery CT, George CF, Houseman TH, Lewis PJ, Turner DM. Absorption and metabolism of nicotine from cigarettes. Br Med J. 1975;4:313–6.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504.
Benowitz NL, Porchet H, Jacob P III. Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog Brain Res. 1989;79:279–87.
Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm. 1994;22:431–45.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94.
Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res. 2007;24:2187–97.
Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90:154–66.
Acknowledgments
We thank the Disease and Therapeutic Response Modeling Program for the Clinical and Translational Sciences Institute (CTSI) at Indiana University. We thank Karmen Dayhuff and Andrea Meyer for assistance with blood draws, Jeff Sturgeon for designing and implementing the custom electronic cigarette, and Ken Mackie for providing facilities to process the blood plasma. Supported by the Indiana Clinical Translational Sciences Institute, CPAC Core facility grant (JWB, DRJ). Supported by the Indiana Clinical and Translational Sciences Institute, funded in part by grant # RR 02576 from the National Institutes of Health, National Center for Research Resources. Analytical work was performed by the Clinical Pharmacology Analytical Core laboratory, a core laboratory of the Indiana University Melvin and Bren Simon Cancer Center supported by the National Cancer Institute grant P30 CA082709. NVM was supported by Eli Lilly and Company through the Indiana Clinical and Translational Sciences Institute (CTSI).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vélez de Mendizábal, N., Jones, D.R., Jahn, A. et al. Nicotine and Cotinine Exposure from Electronic Cigarettes: A Population Approach. Clin Pharmacokinet 54, 615–626 (2015). https://doi.org/10.1007/s40262-014-0221-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0221-7