Abstract
Background and Objective
Quadpill, a single pill containing a quadruple combination of quarter doses of four antihypertensive agents, has been investigated for hypertension treatment. This meta-analysis aims to evaluate the safety and efficacy of quadpill for hypertension management.
Methods
We conducted a systematic review and meta-analysis synthesizing randomized controlled trials evaluating quadpill versus monotherapy or placebo in patients with hypertension, which were retrieved by systematically searching PubMed, EMBASE, Web of Science, SCOPUS, and Cochrane through 17 February, 2023. Continuous and dichotomous outcomes were pooled using mean difference (MD) and risk ratio (RR) along with confidence interval (CI), using Revman Version 5.4 software. Our protocol has been published in PROSPERO with ID: CRD42023406527.
Results
Four randomized controlled trials with a total of 779 patients were included in our analysis. Quadpill was effective in controlling systolic blood pressure in the short term [4–6 weeks] (RR: − 13.00 with 95% CI [− 17.22, − 8.78], p = 0.00001) and in the long term [12 weeks] (RR: − 6.18 with 95% CI [− 9.35, − 3.01], p = 0.0001). Quadpill was also effective in controlling automated diastolic blood pressure in the short term [4–6 weeks] (MD: − 8.15 with 95% CI [− 9.42, − 6.89], p = 0.00001) and in the long term [12 weeks] (MD: − 6.35 with 95% CI [− 10.37, − 2.33], p = 0.002). Moreover, patients in the quadpill group significantly achieved target blood pressure <140/90 (RR: 1.77 with 95% CI [1.26, 2.51], p = 0.001) compared with the control group.
Conclusions
The quadruple ultra-low-dose combination of antihypertensive drugs (quadpill) was effective and safe for hypertension treatment. However, further large-scale, multicenter, randomized controlled trials are still warranted before endorsement in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The quadruple ultra-low-dose combination of antihypertensive drugs (quadpill) was effective and safe for hypertension treatment. |
Further large-scale, multicenter, randomized controlled trials are still warranted to study the long-term effect of the quadpill on hard cardiovascular outcomes, to compare it with recently endorsed antihypertensive drug combinations, and to provide clear long-term data on 24-h ambulatory blood pressure measurements. |
1 Introduction
Hypertension is a chronic disease that is defined as office systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) values ≥ 90 mmHg, as per 2018 European Society of Cardiology/European Society of Hypertension guidelines [1]. However, American College of Cardiology/American Heart Association guidelines released in 2017 defined hypertension as SBP/DBP ≥ 130/80 mmHg [2]. This new grading caused an increase in the prevalence of the disease compared with the old definition of 140/90 mmHg [3]. It was globally estimated that 1.28 billion adults, aged between 30 and 79 years, have been diagnosed with hypertension [4]. Moreover, hypertension is a modifiable risk factor for cardiovascular diseases, and most of the deaths due to hypertension are caused by coronary heart disease and stroke [5, 6].
Hypertension is associated with various microvascular and macrovascular complications, with 10.7 million all-cause mortality cases associated with an SBP ≥ 110–115 mmHg and 7.8 million cases associated with an SBP ≥ 140 mmHg [7, 8]. Uncontrolled hypertension is also associated with various complications, such as stroke, myocardial infarction, and kidney diseases. It is desirable to have a blood pressure (BP) goal of < 140/90 mmHg in hypertensive patients without comorbidities and of < 130 mmHg in high-risk hypertensive patients with high-risk cardiovascular diseases, diabetes mellitus, and chronic kidney disease, as per World Health Organization guidelines released in 2021 [4]. Because of the disease’s chronicity, the intake of antihypertensive drugs with the correct doses over a long period is the basis of hypertension management.
Hypertension control using a combination of different classes of antihypertensive drugs was reported to be more effective than using monotherapy, even in high doses of monotherapy, and the evidence supports that 75% of patients need a combination-based therapy to achieve the BP target [5, 9]. However, several barriers limit the use of combination regimens, such as the psychological factors of the patients and the fear of doctors from the drugs’ adverse effects. Thus, using multiple antihypertensive drugs in reduced doses in the form of one pill can be a better solution to overcome these barriers [10]. The use of one-pill combination therapy was associated with an increase in the patient’s adherence and a more robust BP control compared with the free equivalent combination therapy [10, 11]. Furthermore, in a meta-analysis conducted by Parati et al., the single-pill combination therapy was associated with more significant adherence, compliance, and a reduction in SBP and DBP than the free equivalent combination therapy [12].
Similarly, in a clinical trial conducted by Chow et al. among 21 treatment-naive hypertensive patients in a cross-over design using a quadpill combination of four antihypertensive drugs at quarter doses, it was reported that the quadpill had reduced the ambulatory and office-measured BP [13]. This was subsequently supported by QUARTET, a large randomized controlled trial (RCT) [14]. Although dual ultra-low dose combinations have been shown to reduce adverse events while maintaining efficacy [15], there is a lack of evidence about the exact efficacy and safety of the quadpill. Therefore, the objective of this systematic review and meta-analysis is to summarize the published RCTs that investigated the quadpill containing angiotensin receptor blockers, calcium channel blockerment1s, beta-blockers, and diuretics at a quarter of the standard dose for hypertension management.
2 Methodology
2.1 Protocol Registration
This systematic review and meta-analysis was conducted under the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [16] and the Cochrane Handbook for systematic reviews and meta-analyses [17]. The protocol for this review has been registered and published in PROSPERO with the following ID: CRD42023406527.
2.2 Data Sources and Search Strategy
AB and MA conducted a thorough search for the relevant literature utilizing several databases, including PubMed (MEDLINE), EMBASE, Web of Science, SCOPUS, and the Cochrane Central Register of Controlled Trials (CENTRAL) until 17 February, 2023. No search limitations or filters were used. Further details about the search strategy, including the keywords and search terms, as well as the results of the search, can be found in Table S1 of the Electronic Material (ESM).
2.3 Eligibility Criteria
A PICO criterion was used to include RCTs: population (P): patients with hypertension; intervention (I): quadpill (containing angiotensin receptor blocker, calcium channel blocker, beta-blocker, and a diuretic), at a quarter of the standard dose for hypertension; control (C): monotherapy or placebo; and outcomes (O): primary outcomes: automated SBP and DBP. Among our secondary outcomes are patients who achieved the BP target (140/90 mmHg) and adverse events.
A range of research designs was excluded from our analysis. Specifically, animal studies, pilot studies, various forms of observational studies including cohorts, case-control, cross-sectional, case series, and case reports, single-arm clinical trials, in vitro experiments conducted on tissues and cultures, book chapters, editorials, press articles, publications that only contain abstracts or posters, unpublished study protocols, and studies that were conducted in languages other than English were excluded.
2.4 Study Selection
The review process was carried out using the Covidence online software. Two reviewers (AB and OA) conducted it independently after eliminating any duplicated records. The full texts of the records that met the initial eligibility criteria were examined through full-text screening. Any disagreements were resolved through discussions that included BA.
2.5 Data Extraction
Four reviewers (AB, OA, MMA, and MA) independently used a pre-designed extraction sheet to extract the following data: summary characteristics (study design, country, total participants, quadpill intervention details, control, main inclusion criteria, follow-up duration, and primary outcome); baseline characteristics (number of participants in each group, age, sex, basal metabolic index, patents on baseline monotherapy, SBP, DBP, heart rate, and comorbidities); efficacy data (SBP, DBP, patients who achieved the BP target [140/90 mmHg], and adverse events)
2.6 Risk of Bias and Quality Assessment
Four reviewers (AB, OA, MMA, and MA) assessed the quality of the studies included in the research independently using the Cochrane RoB2 tool [18]. The domains that were evaluated included the risk of bias resulting from the randomization process, the risk of bias due to deviation from the intended intervention, the risk of bias due to missing outcome data, the risk of bias in measuring the outcome, and the risk of bias in selecting the reported results. In the event of any disagreements, the reviewers discussed and resolved them through consensus.
Two evaluators (MA and AB) used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines [19, 20] to assess the quality of evidence. The assessment was conducted for each outcome, and the decisions were supported and reported in a GRADE evidence profile. Any inconsistencies were resolved through discussion.
2.7 Statistical Analysis
The RevMan Version 5.3 software [21] was used to perform the statistical analysis. To combine the outcomes for dichotomous results, the risk ratio was used, while the mean difference (MD) was used for continuous results. Both were calculated with a 95% confidence interval (CI) using the fixed-effects model. However, the random-effects model was used in case of significant heterogeneity (I-square > 50%). The presence and extent of heterogeneity were evaluated using the Chi-square and I-square tests, respectively. Following the Cochrane Handbook (Chapter Nine) [17], heterogeneity was considered significant if the alpha level for the Chi-square test was below 0.1, while the I-square test results were interpreted as follows: not significant for 0–40%, moderate heterogeneity for 30–60%, substantial heterogeneity for 50–90%, and considerable heterogeneity for 75–100%.
3 Results
3.1 Search Results and Study Selection
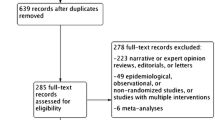
During the search process, a total of 78 studies were identified and evaluated for screening based on their titles and abstracts. After removing 46 duplicates and 23 studies that did not meet the inclusion criteria, nine full-text articles were assessed. Out of these, six were found to be irrelevant and excluded, leaving a total of three eligible RCTs. Finally, four RCTs were included in the qualitative and quantitative analysis after manually adding QURTETE-USA [22] (Fig. 1).
3.2 Characteristics of Included Studies
Four RCTs [13, 14, 22, 23] that met our eligibility criteria were identified, with a total of 779 participants. The studies were conducted in Australia, the USA, and Ireland. Three of the studies were double blinded, and one was single blinded. All studies compared the quadpill containing a combination of a quarter-dose of four antihypertensive drugs with a control group. The duration of treatment ranged from 1 to 12 months. In Chow et al. 2017 and Chow et al. 2021, patients were instructed to take the pill every 24 h at any time but preferably in the morning; thus, there was no fixed relationship between the time of drug intake and the time of BP measurement [13, 14]. In Mahmoud et al., it was reported that BP measurement was obtained 3 h after ingestion of the drug [23], with no details in QUARTET-USA [22]. Ambulatory 24-h BP measurement was assessed every 30 minutes during waking hours and every 60 min during sleep in Chow et al. [13, 14]. Details of the included RCTs, baseline characteristics, and comorbidities of the included participants are reported in Tables 1, 2, and 3, respectively.
3.3 Risk of Bias and Certainty of Evidence
Two RCTs had a low risk of overall bias, while Chow et al. [13] showed some concerns mainly attributed to the unclear risk of reporting bias owing to the lack of a published protocol. Additionally, Mahmud and Feely [23] showed some concerns mainly attributed to the unclear risk of reporting bias owing to the lack of a published protocol and selection bias due to the lack of randomization and allocation concealment details reporting (Fig. 2).
3.4 Primary Outcomes
3.4.1 Automated SBP
The quadpill was effective in controlling automated SBP in the short term [4–6 weeks] (MD: − 13.00 with 95% CI [− 17.22, − 8.78], p < 0.00001) [low-quality evidence] and in the long term [12 weeks] (MD: − 6.18 with 95% CI [− 9.35, − 3.01], p = 0.0001) [moderate-quality evidence] (Fig. 3A, Table 4). Pooled studies were homogenous in the long term (p = 0.25, I2 = 26%) but heterogeneous in the short term (p = 0.007, I2 = 75%). Heterogeneity was not resolved by a sensitivity analysis (Table S2 of the ESM).
3.4.2 Automated DBP
The quadpill was effective in controlling DBP in the short term [4–6 weeks] (MD: − 8.15 with 95% CI [− 9.42, − 6.89], p < 0.00001) [high-quality evidence] and in the long term [12 weeks] (MD: − 6.35 with 95% CI [− 10.37, − 2.33], p = 0.002) [low-quality evidence] (Fig. 3B, Table 4). Pooled studies were homogenous in the short term (p = 0.30, I2= 18%) but heterogeneous in the long term (p = 0.06, I2 = 71%). Heterogeneity was not resolved by a sensitivity analysis (Table S2 of the ESM).
3.5 Secondary Outcomes
3.5.1 Target BP (140/90 mmHg) [4–6 Weeks]
More patients in the quadpill group significantly achieved the BP target < 140/90 (RR: 1.77 with 95% CI [1.26, 2.51], p = 0.001) [moderate-quality evidence], compared with the control group (Fig. 3C; Table 4). Pooled studies were heterogeneous (p = 0.10, I2 = 57%). The heterogeneity was not resolved by a sensitivity analysis (Table S2 of the ESM).
3.5.2 Adverse Events
There was no difference between the quadpill and the control groups regarding the safety outcomes, including the incidence of any adverse events (RR: 1.36 with 95% CI [0.86, 2.15], p = 0.19) [low-quality evidence], any serious adverse events (RR: 1.13 with 95% CI [0.56, 2.25], p = 0.74) [low-quality evidence], and any adverse events leading to drug discontinuation (RR: 0.89 with 95% CI [0.19, 4.11], p = 0.88) [very low-quality evidence] (Fig. 4; Table 4). Pooled studies were heterogeneous regarding any adverse events leading to drug discontinuation (p = 0.07, I2= 63%). The heterogeneity was not resolved by a sensitivity analysis (Table S2 of the ESM).
4 Discussion
This comprehensive meta-analysis, including RCTs, tests the efficacy and safety of the quadpill containing four antihypertensive drugs from the respective classes of ACE inhibitors/angiotensin receptor blockers, calcium channel blockers, beta-blockers, and a diuretic at a quarter dose to the conventional approach of utilizing monotherapy for antihypertensive. As per this study, quadpill was more effective in controlling automated SBP and DBP in the short term and the long term. Furthermore, a similar benefit was observed in achieving a BP target (< 140/90) over the follow-up of 4–6 weeks in the quadpill group compared with the control. Finally, there was no statistically significant difference between the quadpill and the control groups regarding the important safety outcomes, such as the incidence of any adverse events, any serious adverse events, and any adverse events leading to drug discontinuation.
In most patients who have stage 1 or stage 2 hypertension, current US guidelines recommend initiating monotherapy with further titration or adding other agents if needed to achieve the BP target [2]. Per our study, the quadpill is superior in lowering and reaching the BP target compared with monotherapy. The observed effect is likely multifactorial, involving drug pharmacokinetics, an additive drug effect on BP reduction, patient- and physician-related factors, and environmental factors. Most of the therapeutic effects of antihypertensive drugs are achieved by 50% of their maximum dose. Multiple studies have shown that multi-drug combination therapy is superior in lowering BP compared with doubling the dose of monotherapy [13, 24, 25]. To clarify, it has been observed that a combination of two drugs at a quarter dose each can result in a reduction of 10 mmHg in SBP [25]. Additionally, a combination of four drugs at a quarter dose each has shown a greater reduction of 20 mmHg in SBP compared with a placebo [13]. Additionally, Wald et al. reported that combining two drugs from the four classes, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, thiazide, and calcium channel blockers, produced an additive BP-lowering effect nearly five times greater than doubling the dose of monotherapy [9].
There has been a paradigm shift in using combination therapy in recent years. US Joint National Committee guidelines recommend initiating therapy using two agents where BP control > 20 mmHg above the systolic or 10 mmHg above the diastolic goal is required [2]. Similarly, American College of Cardiology/American Heart Association 2017 guidelines recommend initiating single-pill combination therapy in patients with BP >20/10 mmHg above the BP goal. European Society of Cardiology/European Society of Hypertension guidelines recommend a similar approach in patients with BP > 140/90 mmHg [1, 2].
The earlier achievement of BP control is vital as it is an important determinant of cardiovascular outcomes [26]. For example, Karmali et al. [27] reported that an SBP reduction of 10 mmHg reduces all-cause mortality by 11% and coronary heart disease by 16% [26]. However, another large study showed alarming data, as nearly 50% of the US adult population with known hypertension had sub-optimal BP control [26]. In our study, the quadpill was significantly better in achieving the target BP on follow-up visits when compared with the control group. This suggests that the quadpill strategy can effectively reach and maintain the long-term target BP goal. Furthermore, studies have also shown that multi-drug combinations resulting in lower numbers of pills needed to be taken improved patient adherence to the medical regimen and reduced the need for repeat visits to achieve desirable BP control, and reduced healthcare-related expenditure [28, 29]. However, further studies are warranted to determine the long-term impact on hard cardiovascular outcomes.
In clinical practice, many physicians are cautious in starting fixed-dose combination pills, especially in certain high-risk populations, including the geriatric population or patients at risk of orthostatic hypotension and recurrent falls. Dual ultra-low-dose combinations have been used historically to reduce adverse effects while maintaining the efficacy of monotherapy [30]. However, according to our meta-analysis, the quadpill has not shown any significant difference regarding the adverse events in treatment versus control groups. It is important to note that there can be considerable variations in individual responses to antihypertensive agents. This variability is likely influenced by the patient’s demographics, including age, sex, and ethnicity. Therefore, an individualized approach considering the patient’s demographic factors and preferences, resulting in shared decision making, would be a reasonable approach while initiating the antihypertensive regimen.
5 Strengths and Limitations
This meta-analysis was conducted strictly following the methodology guidelines provided by PRISMA and Cochrane to ensure rigorous findings that can be considered the highest level of evidence in this regard. However, it is important to note that our meta-analysis has a few limitations. First, only four RCTs with a relatively small number of patients were included, limiting our findings’ generalizability. Second, Chow et al. [13] was a cross-over placebo-controlled trial, compared with the other RCTs, monotherapy controlled. However, it only included 18 patients with a small weight and effect on our data. Third, the follow-up duration was not long enough to determine the long-term efficacy of quadpill and its effect on hard cardiovascular outcomes. Fourth, QUARTET-USA was only presented at the American Heart Association scientific sessions in 2022, and it was not published in a peer-reviewed publication [22]. Finally, a quarter dose may not necessarily represent the optimal dose, given that every drug has different pharmacodynamics, mode of action, and dose–response curves. It is hard to predict whether all four included agents in the pill contribute equally to its efficacy or if this combination is superior to dual combination pills already being used in clinical practice. Hence, further research should assess the comparative efficacy and tolerability compared with other combined regimens, especially the initial dual combination therapy now recommended for most patient groups in recent guidelines [2, 15].
6 Conclusions
Despite a quadruple ultra-low-dose combination of antihypertensive drugs (quadpill) being effective and safe for hypertension treatment, current evidence is based on a few small RCTs. Therefore, further large-scale multicenter RCTs are still warranted to study the long-term effect of the quadpill on hard cardiovascular outcomes, compare it with recently endorsed antihypertensive drug combinations, and provide clear long-term data on 24-h ambulatory BP measurements, taking into consideration that drugs in a quarter dose have no significant effect at the end of 24 h and also some drugs work better during the awake hours and others while asleep.
References
Williams B, Mancia G, Spiering W, ESC Scientific Document Group, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. https://doi.org/10.1093/eurheartj/ehy339.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2018;138:e484-594.
Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–18.
WHO. Hypertension. Available from: https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed 9 Mar 2023.
Al-Makki A, DiPette D, Whelton PK, Murad MH, Mustafa RA, Acharya S, et al. Hypertension pharmacological treatment in adults: a World Health Organization guideline executive summary. Hypertension. 2022;79:293–301.
Abuelazm M, Saleh O, Albarakat MM, Katamesh B, Abdalshafy H, Mahmoud A, et al. The effect of bedtime versus morning dosing of antihypertensive drugs on the cardiovascular outcomes: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2023;41:1595–605.
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37.
Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165–82.
Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300.
Grassi G, Mancia G. Quarter dose quadpill combinations: a new therapeutic approach. Nat Rev Nephrol. 2017;13:266–7.
Kotsis V, Stabouli S, Bouldin M, Low A, Toumanidis S, Zakopoulos N. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension. 2005;45:602–7.
Parati G, Kjeldsen S, Coca A, Cushman WC, Wang J. Adherence to single-pill versus free-equivalent combination therapy in hypertension. Hypertension. 2021;77:692–705.
Chow CK, Thakkar J, Bennett A, Hillis G, Burke M, Usherwood T, et al. Quarter-dose quadruple combination therapy for initial treatment of hypertension: placebo-controlled, crossover, randomised trial and systematic review. Lancet. 2017;389:1035–42. https://doi.org/10.1016/S0140-6736(17)30260-X.
Chow CK, Atkins ER, Hillis GS, Nelson MR, Reid CM, Schlaich MP, et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double-blind, active-controlled trial. Lancet. 2021;398:1043–52.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–57.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:I4898.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. Rating quality of evidence and strength of recommendations: what is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Rating quality of evidence and strength of recommendations: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924.
RevMan. Cochrane training. Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. Accessed 3 Aug 2021.
Efficacy and safety of a quadruple ultra-low-dose treatment for hypertension. 2022. Available from: https://clinicaltrials.gov/show/NCT03640312. Accessed 23 Sep 2023.
Mahmud A, Feely J. Low-dose quadruple antihypertensive combination: more efficacious than individual agents: a preliminary report. Hypertension. 2007;49:272–5.
Salam A, Atkins ER, Hsu B, Webster R, Patel A, Rodgers A. Efficacy and safety of triple versus dual combination blood pressure-lowering drug therapy: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37:1567–73.
Salam A, Huffman MD, Kanukula R, Hari Prasad E, Sharma A, Heller DJ, et al. Two-drug fixed-dose combinations of blood pressure-lowering drugs as WHO essential medicines: an overview of efficacy, safety, and cost. J Clin Hypertens. 2020;22:1769–79.
Weber MA, Julius S, Kjeldsen SE, Brunner HR, Ekman S, Hansson L, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE trial. Lancet. 2004;363:2049–51.
Karmali KN, Lloyd-Jones DM, Berendsen MA, Goff DC, Sanghavi DM, Brown NC, et al. Drugs for primary prevention of atherosclerotic cardiovascular disease: An overview of systematic reviews. JAMA Cardiol. 2016;1:341–9.
Mallat SG, Tanios BY, Itani HS, Lotfi T, Akl EA. Free versus fixed combination antihypertensive therapy for essential arterial hypertension: a systematic review and meta-analysis. PLoS ONE. 2016;11: e0161285.
Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407.
Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Conflict of interest
Mohamed Abuelazm, Shafaqat Ali, Othman Saleh, Amr Badr, Obieda Altobaishat, Majd M. AlBarakat, Aya Aboutaleb, Abdelmonem Siddiq, and Basel Abdelazeem have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data used in this meta-analysis are included in the article and the supplementary material.
Code availability
Not applicable.
Author contributions
MA conceived the idea. MA and AB designed the research workflow. AB and MA searched the databases. AB, OA, OS, MMA, and AA screened the retrieved records, extracted relevant data, and assessed the quality of evidence, and AB resolved the conflicts. MA and OS performed the analysis. MA, SA, OS, and AS wrote the final manuscript. AB supervised the project. All authors have read and agreed to the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Abuelazm, M., Ali, S., Saleh, O. et al. The Safety and Efficacy of Quadruple Ultra-Low-Dose Combination (Quadpill) for Hypertension Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin Drug Investig 43, 813–826 (2023). https://doi.org/10.1007/s40261-023-01313-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01313-3