Abstract
Acthar® Gel (repository corticotropin injection) is a naturally sourced complex mixture of adrenocorticotropic hormone analogs and other pituitary peptides that is believed to have both steroidogenic and nonsteroidogenic immunomodulatory effects via activation of melanocortin receptors in various cells throughout the body. Since 1952, Acthar has been approved by the US Food and Drug Administration to treat a variety of autoimmune and inflammatory diseases. Since 2014, Mallinckrodt Pharmaceuticals has conducted a large number of preclinical, clinical, and real-world-evidence studies of Acthar for the treatment of rheumatoid arthritis, systemic lupus erythematosus, dermatomyositis and polymyositis, multiple sclerosis relapse, ophthalmic disorders, sarcoidosis, and nephrotic syndrome. To date, Acthar has been the subject of more than 500 publications, many of which demonstrate the safety and efficacy of Acthar in patients with inflammatory diseases for whom standard treatments were ineffective or intolerable. Here, we review the history of Acthar and the findings of studies that have investigated the mechanism of action, safety, efficacy, and real-world effectiveness of Acthar for the treatment of inflammatory diseases.
Plain Language Summary
Acthar® Gel is an anti-inflammatory drug that directly affects the immune system in a manner that differs from other anti-inflammatory drugs, such as corticosteroids. Since 1952, Acthar has been approved by the U.S. Food and Drug Administration to treat a variety of diseases involving inflammation. The commercial rights to produce Acthar have changed hands several times over the years, beginning with Armour Pharmaceuticals and most recently ending with Mallinckrodt Pharmaceuticals in 2014. Since then, Mallinckrodt has conducted multiple studies in animals to demonstrate the function of Acthar compared with other anti-inflammatory drugs. Further, several clinical trials in humans and studies of hospital or clinical practice records have confirmed the safety and effectiveness of Acthar as a treatment for many inflammatory diseases.
AbstractSection Infographic
A podcast discussion by the authors on Acthar® Gel treatment for patients with autoimmune and inflammatory diseases, including their own personal reflections and experiences with Acthar® Gel. For a transcript of the podcast see the electronic supplementary material. (MP4 177883 kb)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acthar® Gel is an anti-inflammatory drug that directly affects the immune system in a manner that differs from other anti-inflammatory drugs, such as corticosteroids. |
Since 1952, Acthar has been approved by the US Food and Drug Administration to treat a variety of diseases involving inflammation; since 2014, Mallinckrodt Pharmaceuticals has conducted multiple studies in animals to demonstrate the function of Acthar compared with other anti-inflammatory drugs. |
Furthermore, several clinical trials in humans and studies of hospital or clinical practice records have confirmed the safety and effectiveness of Acthar as a treatment for many inflammatory diseases. |
1 Introduction
Acthar® Gel (repository corticotropin injection) is a naturally sourced complex mixture of adrenocorticotropic hormone (ACTH) analogs and other pituitary peptides [1], derived from the entire porcine pituitary gland. The Acthar mixture is solubilized in a 16% gelatin formulation designed for prolonged release after subcutaneous or intramuscular injection [2, 3]. A major active component is N-25 deamidated, full-length porcine ACTH1–39 [3]. ACTH is cleaved from its pro-opiomelanocortin prohormone, as are other melanocortin signaling peptides, including α-melanocyte-stimulating hormone (α-MSH), β-MSH, and γ-MSH, all of which have been shown to have anti-inflammatory effects [4,5,6,7,8,9].
Acthar is believed to have anti-inflammatory and immunomodulatory effects by activating melanocortin receptors (MCRs) in various cell types throughout the body [10]. Although Acthar can stimulate the adrenal cortex to induce steroidogenesis, studies in healthy human subjects have demonstrated substantially lower cortisol production with Acthar compared with synthetic ACTH1–24 depot, at amounts slightly above normal endogenous levels [3, 11]. An 80 U subcutaneous dose of Acthar produced mean peak total cortisol (Emax) levels of 22.2 µg/dL at 24 h [11].
Analysis of Acthar binding and activation of all 5 MCRs demonstrated a distinct activity profile compared with synthetic MCR agonists [12]. Acthar’s lowest full agonistic activity is at MC2R, which is expressed in adrenocortical cells to promote steroidogenesis, whereas synthetic ACTH1–24 has its highest activity at MC2R. This was consistent with substantially less endogenous corticosteroid production in rats and humans exposed to Acthar compared with synthetic ACTH1–24 [11, 12]. The relatively low endogenous cortisol production by Acthar eliminates the need for cortisol level testing or hypersensitivity skin tests during clinical use [3, 11, 12].
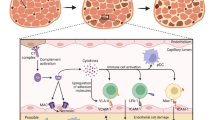
Acthar has been shown to exert a direct immunomodulatory effect on B cells and macrophages, independent from its steroidogenic activity [13, 14]. The expression of genes involved in B-cell proliferation and function were downregulated in response to Acthar [15]. This effect was not recapitulated by individual pituitary neuropeptides, including ACTH1–39, ACTH1–24, and α-MSH [15]. In addition, Acthar was shown to inhibit B-cell activation and antibody production via suppression of toll-like receptor (TLR) 9 and B-cell receptor engagement [16]. Inflammatory cytokine production from human macrophages was reduced in vitro by Acthar in a steroid-independent manner [14]. Moreover, in a mouse model of relapsing-remitting multiple sclerosis (MS), Acthar inhibited inflammation and demyelination within the spinal cord and suppressed ex vivo myelin peptide-induced CD4+ T-cell proliferation [17]. Taken together, these studies confirm a different mechanism of action for Acthar compared with other anti-inflammatory drugs (electronic supplementary material [ESM] Fig. S1).
2 History of Acthar
Acthar was initially developed by Armour Pharmaceuticals and was approved by the US Food and Drug Administration (FDA) in 1952 for many indications [18], including connective tissue diseases (e.g., systemic lupus erythematosus [SLE], psoriatic arthritis, and rheumatoid arthritis [RA]), hypersensitivities (contact dermatitis, severe asthma, and hay fever), acute inflammatory disorders of the eye or skin, proteinuria in nephrotic syndrome, and many other inflammatory diseases. The initial approval was prior to the Kefauver Harris Amendment of 1962 that required drug manufacturers to provide evidence of efficacy prior to FDA approval [2, 19]. A Drug Efficacy Study Implementation review conducted between 1971 and 1977 concluded that Acthar was effective for 52 indications, and the label was expanded accordingly. Following the Drug Efficacy Study Implementation review, the approved indications included numerous disorders such as endocrine, opthalmic, respiratory, and dermatologic diseases, as well as allergic conditions.
In 1979, Acthar was approved by the FDA for the treatment of MS exacerbations in adults [18]. Due to development of corticosteroid therapies, Acthar fell out of favor in the 1980s for some conditions and was largely replaced by prednisone for many indications [20]. In 1985, Rorer Pharmaceuticals acquired the rights to Acthar, and the company later merged with Rhone-Poulenc to form Rhone-Poulenc Rorer. There was a shortage of Acthar in the mid-1990s, as Rhone-Poulenc Rorer had chosen to discontinue the product. However, in response to requests from physicians and patient groups, a limited amount of Acthar was produced, although demand still far exceeded the supply [20]. The complex and expensive purification process became unsustainable, and Aventis (which was formed in 1999 from a merger of Rhone-Poulenc Rorer and Hoechst) sold the rights to Acthar to Questcor Pharmaceuticals in 2001 [20].
Multiple research studies were initiated and published by Questcor, which helped characterize Acthar’s mechanism of action [21,22,23,24]. One such study provided evidence of Acthar’s immunomodulatory activity and related efficacy in a mouse model of SLE [24]. In a transgenic mouse model of amyotrophic lateral sclerosis, Acthar was found to delay symptoms of disease onset and reduce the levels of toxic superoxide dismutase both in the mouse and in cultured fibroblasts [22]. Arnason et al. described the corticosteroid-independent effects of Acthar and other melanocortins relevant for clinical management of MS [21]. Similarly, Berkovich et al. presented a review to further characterize the complex and dynamic mechanism of action of Acthar via the MCR system in MS exacerbations [23].
For many years, Acthar was used as first-line therapy for infantile spasms, even though it was not an FDA-approved indication. Baram et al. demonstrated that a 2-week course of Acthar (150 U/m2/day) was more effective than prednisone (2 mg/kg/day) in the suppression of spasms and hypsarrhythmia [1, 25]. A positive response was reported in 86.6% of patients who achieved cessation of spasms and elimination of hypsarrhythmia with no discontinuation or modification of the therapy [25]. In 2004, the American Academy of Neurology and the Child Neurology Society reported that ACTH is likely effective for the short-term treatment of infantile spasms and resolution of hypsarrhythmia, although there was insufficient evidence to recommend an optimal dosage or duration of treatment [26]. Questcor invested in research and development to attain FDA approval of Acthar for the management of infantile spasms in 2010 [20]. In addition, Questcor agreed to remove 33 of the previously approved indications to conform with modern labeling requirements, leaving a total of 19 indications (ESM Table S1) [1, 27]. An updated 2012 report by the American Academy of Neurology and the Child Neurology Society reinforced the use of Acthar in treating infantile spasms [28].
Mallinckrodt Pharmaceuticals purchased Questcor in 2014, focused on modernizing the manufacturing of Acthar, and initiated research activities to further evaluate the safety and efficacy of the drug (Fig. 1) [29]. Substantial data about Acthar have been generated from at least 20 preclinical mechanistic studies, more than 50 real-world evidence/health economics and outcomes research studies, and 8 clinical studies targeting approximately 900 enrolled patients [29]. To date, more than 500 manuscripts and abstracts have been published regarding Acthar [29].
In 2021, the Acthar label was changed to provide up-to-date safety information and remove the statement: “common adverse reactions for Acthar Gel are similar to those of corticosteroids” on the basis of extensive pharmacovigilance and clinical trial data [30, 31]. Based on analysis of decades of pharmacovigilance data, the most commonly reported adverse effects related to Acthar use include injection site reaction, fatigue, fluid retention, insomnia, headache, high blood glucose levels, hypertension, increased risk of infections, and irritability [1, 30].
3 Clinical Studies of Acthar: 2014–2022
3.1 Rheumatoid Arthritis
The use of Acthar for the treatment of RA was assessed in a randomized, placebo-controlled withdrawal trial [32]. No new or unexpected safety signals were observed, and more than 60% of patients achieved low disease activity by week 12 of therapy [32]. Most patients maintained low disease activity during 12 additional weeks of Acthar therapy and for 3 months following discontinuation of the drug. Correlations between patient-reported outcomes and clinical response to Acthar were investigated using data from the clinical trial [33]. Clinical responses, including Disease Activity Score with 28-Joint Count and erythrocyte sedimentation rate, total joint count, and Clinical Disease Activity Index, were found to directly correlate with patient-reported outcomes [33]. Predictors of positive response to Acthar in patients with RA included lower baseline levels for swollen joint count, total joint count, Erythrocyte Sedimentation Rate, and Clinical Disease Activity Index, as well as shorter RA duration [34]. Osteoarthritis and other joint-related conditions were predictive of negative response to Acthar [34].
A claims database analysis indicated that Acthar was initiated in patients with RA who had more severe disease, comorbidities, and concomitant use of treatments including disease-modifying antirheumatic drugs (DMARDs), corticosteroids, opioids, and nonsteroidal anti-inflammatory drugs [35]. Real-world treatment patterns for patients with RA evaluated from medical records revealed that Acthar improved clinical outcomes and lowered the need for concomitant therapies up to 1 year after initiation of treatment [36, 37]. Busch et al. reported improvements in multiple RA disease assessments 12 months after Acthar initiation, as well as reductions in opioid, glucocorticoid, and nonsteroidal anti-inflammatory drug use; a small proportion (4.3%) of patients discontinued Acthar due to adverse effects (Table 1) [37].
In an analysis of safety data from a randomized clinical trial of Acthar used as an adjunctive therapy for RA (along with DMARDs and glucocorticoids), adverse events (AEs) in the Acthar cohort were compared with those reported in 4 other randomized trials using low-dose glucocorticoids/DMARDs (without Acthar) [31]. Ten of the 16 most frequent AEs (in ≥ 1% of patients) associated with glucocorticoids/DMARD treatment occurred at a lower rate in patients treated with Acthar. These included RA flare, arthralgia, abdominal pain/gastrointestinal symptoms, nasopharyngitis, insomnia, flushing, bronchitis, chest pain, depression, and vertigo. It was concluded that there was no additional risk with the use of Acthar as an add-on therapy to low-dose glucocorticoids and DMARDs for the management of RA [31].
3.2 Systemic Lupus Erythematosus
A single-site, 4-week, open-label trial using Acthar for the treatment of moderate to severe SLE noted significant improvements in SLE Disease Activity Index-2000 scores in patients with refractory disease [38]. No treatment-related serious or unexpected AEs were reported. A 6-month extension of the trial found significant improvements in SLE Disease Activity Index-2000 scores and decreased swollen, tender, and total joint counts through 6 months following initiation of Acthar [39].
A phase IV pilot study of Acthar for the treatment of persistent SLE noted a trend of better response in the Acthar group versus the placebo group after 8 weeks of treatment, as well as statistically significant improvement for several secondary endpoints [40]. The incidence of AEs was similar between cohorts, and Acthar was well tolerated. A post hoc analysis demonstrated durable efficacy over 52 total weeks of Acthar treatment, including an open-label extension of the pilot study. No new safety signals were identified during the 44 weeks of open-label extension [41].
In a phase IV, placebo-controlled, randomized clinical trial, the proportion of Acthar responders as assessed by the SLE Responder Index 4 did not achieve statistical significance between Acthar and placebo [42]. However, the SLE Responder Index 4 may not have been sufficiently sensitive to detect partial improvement and may only distinguish complete resolution of a symptom. Acthar treatment did show disease improvement in several secondary efficacy assessments. No new safety signals or unexpected AEs were reported. Patient-reported outcomes indicated that Acthar provided improvements in quality of life and work productivity versus placebo over the 24-week trial [43]. Biomarker analysis revealed that Acthar reduced inflammation through B-cell immunomodulation (Table 2) [44].
A narrative review found that pooled data from 3 clinical trials of SLE showed significant improvement of British Isles Lupus Assessment Group 2004 index scores after 8 weeks of Acthar therapy, and decreased tender and swollen joint counts after 4 weeks. Few serious AEs were reported in the trials and Acthar exhibited an acceptable safety profile [45].
3.3 Dermatomyositis and Polymyositis
An observational registry study of dermatomyositis and polymyositis found that Acthar therapy resulted in an improved clinical course in 14/24 patients, suggesting that it is an effective option for refractory dermatomyositis/polymyositis. Mild to moderate AEs were reported in 10 patients, including worsening diabetes in some patients [46].
Seven of 9 patients who completed 24 weeks of Acthar treatment in an open-label study of refractory dermatomyositis showed improvements in Cutaneous Dermatomyositis Disease Area and Severity Index and Physician’s Global Assessment scores [47]. AEs were mild and no patients stopped medication during the study [47]. Another study also noted improvement of refractory dermatomyositis/polymyositis in 70% of patients during 24 weeks of Acthar therapy, with a significant decrease in steroid dose [48]. Four serious treatment-related AEs occurred (2 of which were due to herpes zoster), but overall, Acthar was considered to be well tolerated [48].
A real-world profile of Acthar usage patterns and outcomes showed improvement of dermatomyositis/polymyositis in 66.7% of patients, based on a medical records review. In this observational study, 25% of patients with dermatomyositis/ polymyositis had treatment-related AEs that required discontinuation (Table 3) [49].
3.4 Multiple Sclerosis Relapse
Results of an observational prospective registry study of MS relapse found that patients treated with Acthar showed significant improvements in disease assessment scores, including the MS Impact Scale Version 1, the Clinical Global Impression of Improvement, and Work Productivity and Activity Impairment Questionnaire: MS at 2- and 6-month timepoints [50]. A follow-up analysis of the registry study showed that American Academy of Neurology quality metrics guidelines were followed by health care professionals during Acthar treatment in patients with MS relapse, and Acthar was associated with improved outcomes in the domains of disability, fatigue, cognitive impairment, depression, and quality of life [51].
A randomized controlled trial for treatment of MS relapse that inadequately responded to corticosteroids showed greater response/improvement for patients who received Acthar versus placebo at days 7, 21, and 42 on the Expanded Disability Status Scale and Clinical Global Impression of Improvement. No serious AEs or deaths were reported. It was concluded that Acthar was effective for patients with MS that was not responsive to corticosteroid treatment (Table 4) [52].
3.5 Ocular Cicatricial Pemphigoid
A case study of a patient with ocular cicatricial pemphigoid who was treated for 19 months with Acthar demonstrated significant improvement of ocular surface inflammation in conjunction with tapering of systemic steroid use. No serious AEs were observed (Table 5) [53].
3.6 Optic Neuritis
In an open-label, single-arm study of acute demyelinating optic neuritis, patients who began Acthar therapy within 2 weeks of symptom onset and were treated for 15 days showed improvements in visual acuity tests over the course of 400 days following onset of disease [54]. Visual acuity improvements were noted for the eye affected with optic neuritis, as well as the contralateral eye, and it was concluded that further studies exploring the potential efficacy of Acthar were warranted. Two serious treatment-related AEs were reported, including an allergic reaction and an MS relapse (Table 5) [54].
3.7 Uveitis
A medical records review of uveitis noted that 84% of patients who had begun Acthar therapy within the previous 12 months had improvement of vision. Concomitant medications were reduced during Acthar therapy and no patients had worsening disease (Table 5) [55].
3.8 Retinal Vasculitis
In a prospective, open-label, single-arm study, patients with noninfectious vasculitis showed improvement in retinal vasculitis severity scoring in 16 of 30 eyes following 12 weeks of Acthar therapy. One patient discontinued treatment due to an injection site reaction, but Acthar was well tolerated overall and considered a potentially effective therapy (Table 5) [56].
3.9 Noninfectious Keratitis
A phase IV open-label study of noninfectious keratitis showed disease improvements following 12 weeks of Acthar treatment, as determined by assessments for dry eye, ocular discomfort, and pain [57]. Acthar was determined to be effective, with no new safety signals identified. Similarly, a pilot study of Acthar for dry eye disease found significant improvements via assessments of erythema and superficial punctate keratitis lesions using either corneal fluorescein or lissamine green staining. Symptom Assessment in Dry Eye scores also showed improvement with Acthar. No ocular AEs were observed and intraocular pressure significantly decreased by day 84 of Acthar treatment (Table 5) [58].
3.10 Ocular Mucous Membrane Pemphigoid
A chart review evaluated treatment with Acthar in patients with refractory ocular mucous membrane pemphigoid that did not respond to immunomodulatory drugs. Acthar treatment for 21 months was associated with controlled disease and was considered a viable alternative or adjunctive therapy, with no serious drug-related AEs observed (Table 5) [59].
3.11 Sarcoidosis
Among patients with sarcoidosis who were treated with Acthar for 3 months or longer in a chart review study, 38% experienced significant clinical improvement and 89% who were receiving prednisone at initiation of treatment had their prednisone doses reduced by more than 50% [60]. Of the patients who began the study, 23% discontinued due to drug toxicity [60]. A multicenter, single-blind trial of chronic pulmonary sarcoidosis noted a significant decrease in prednisone dose as well as improvements in diffusing capacity of the lungs for carbon monoxide, King’s Sarcoidosis Questionnaire general health status, and Fatigue Activity Scale score in patients who completed 24 weeks of Acthar treatment [61].
An analysis of medical records from patients with sarcoidosis treated with Acthar indicated that it was a viable treatment for advanced sarcoidosis, with overall status improvement in 95% of patients. The percentage of patients using corticosteroids was reduced from 61.3% at initiation of Acthar to 12.9% after 3 months of therapy (Table 6) [62].
A Delphi study on the management of Acthar for pulmonary sarcoidosis made key recommendations, including an initial Acthar regimen at a lower dose (40 U) twice weekly for less severe disease, followed by an individualized maintenance dose for responders [63]. However, the panelists were unable to reach a consensus on whether a higher initial Acthar dose should be used for more severe disease [63]. In addition, the use of concomitant steroids should quickly be tapered, and guidance for AE management was presented. Furthermore, a recent narrative review provided a detailed overview of the clinical guidelines, mechanism of action, efficacy, and safety of Acthar in the treatment of pulmonary sarcoidosis [10].
PULSAR, a recently completed phase IV exploratory clinical trial for pulmonary sarcoidosis, examined efficacy endpoints such as steroid tapering, pulmonary function tests, lung imaging, patient-reported outcomes, and a novel composite sarcoidosis treatment score during 48 weeks of Acthar therapy [64, 65]. The study showed trends in efficacy data that suggested greater improvements with Acthar compared with placebo, including faster discontinuation of corticosteroids [65].
3.12 Nephrotic Syndrome
Substantial improvement was noted for patients with nephrotic syndrome due to idiopathic membranous nephropathy who were treated with Acthar in a phase Ib/II open-label, dose-finding study. By 12 months of follow-up, significant reductions in proteinuria and improvements in serum albumin and cholesterol levels were observed, with increased improvements associated with higher cumulative Acthar dose and no significant AEs reported [66].
In a case series of nephrotic syndrome due to various etiologies, Acthar treatment was associated with proteinuria reduction of 30% or greater in more than 80% of patients. About 16% of patients discontinued the treatment due to AEs that included weight gain, hypertension, edema, fatigue, and seizures [67].
In another case series, 53.8% of patients with nephrotic syndrome due to minimal change disease or focal segmental glomerulosclerosis showed a complete or partial response to Acthar as assessed by reductions in protein-to-creatinine ratio. Five patients from the focal segmental glomerulosclerosis group exhibited steroid-like adverse effects, which resulted in the discontinuation of 2 patients from Acthar therapy [68].
The safety and efficacy of Acthar alone or in combination with tacrolimus for focal segmental glomerulosclerosis or membranous nephropathy were examined in a prospective, open-label trial. Proteinuria was significantly reduced after 6 months with Acthar alone. In patients with no response or partial response to Acthar, an additional 6-month treatment with a combination of Acthar and tacrolimus improved the clinical response compared with Acthar alone. All patients completed at least 6 months of therapy and none required discontinuation of Acthar [69].
In a chart-review study, Acthar for the treatment of focal segmental glomerulosclerosis following kidney transplant was evaluated in patients who had received donor kidneys. Complete or partial remission of focal segmental glomerulosclerosis was observed in 36% of patients after receiving Acthar therapy for 6 to 24 months. No patients discontinued Acthar due to AEs [70].
An open-label pilot study of immunoglobulin (Ig) A nephropathy found significantly reduced proteinuria, stable glomerular filtration rate, and increased serum albumin in patients with severe disease at 12-month follow-up following 6 months of Acthar treatment. AEs included 2 viral infections (1 herpes zoster, 1 upper respiratory tract) and 4 bacterial infections (2 sinusitis, 1 pneumonia, 1 otitis media) (Table 7) [71].
4 Conclusions
Acthar has a long-standing history of treatment of autoimmune and other inflammatory disorders, with extensive clinical data confirming its safety and efficacy in many therapeutic areas. Early-phase clinical and preclinical studies have supported a distinct mechanism of action compared with standard of care therapies. Mallinckrodt Pharmaceuticals has conducted more than 20 preclinical mechanistic studies of Acthar and initiated 8 clinical studies with a targeted combined enrollment of approximately 900 patients [29]. In addition, the use of Acthar has been the subject of more than 500 published manuscripts and abstracts to date [29]. The results of these studies support the safety and efficacy of Acthar in patients with inflammatory diseases for whom standard treatments have become ineffective or are associated with intolerable adverse effects.
References
Acthar Gel. Package insert. Mallinckrodt Pharmaceuticals; 2021. https://www.acthar.com/Static/pdf/Acthar-PI.pdf. Accessed 16 Dec 2022.
Philbin M, Niewoehner J, Wan GJ. Clinical and economic evaluation of repository corticotropin injection: a narrative literature review of treatment efficacy and healthcare resource utilization for seven key indications. Adv Ther. 2017;34(8):1775–90. https://doi.org/10.1007/s12325-017-0569-9.
Wang X, Pham L, Poola N, Brooks LR, Due B. Comparison of steroidogenic exposure following the administration of repository corticotropin injection with a synthetic ACTH1-24 depot and methylprednisolone in healthy subjects. Clin Pharmacol Drug Dev. 2021;10(7):777–88. https://doi.org/10.1002/cpdd.894.
Zhang C, Chery S, Lazerson A, Altman NH, Jackson R, Holt G, et al. Anti-inflammatory effects of alpha-MSH through p-CREB expression in sarcoidosis like granuloma model. Sci Rep. 2020;10(1):7277. https://doi.org/10.1038/s41598-020-64305-9.
Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29(5):581–602. https://doi.org/10.1210/er.2007-0027.
Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. Sci World J. 2010;10:1840–53. https://doi.org/10.1100/tsw.2010.173.
Getting SJ, Gibbs L, Clark AJ, Flower RJ, Perretti M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J Immunol. 1999;162(12):7446–53. https://doi.org/10.4049/jimmunol.162.12.7446.
Kadiri JJ, Thapa K, Kaipio K, Cai M, Hruby VJ, Rinne P. Melanocortin 3 receptor activation with [D-Trp8]-gamma-MSH suppresses inflammation in apolipoprotein E deficient mice. Eur J Pharmacol. 2020;880: 173186. https://doi.org/10.1016/j.ejphar.2020.173186.
Lonati C, Gatti S, Catania A. Activation of melanocortin receptors as a potential strategy to reduce local and systemic reactions induced by respiratory viruses. Front Endocrinol (Lausanne). 2020;11: 569241. https://doi.org/10.3389/fendo.2020.569241.
Mirsaeidi M, Baughman RP. Repository corticotropin injection for the treatment of pulmonary sarcoidosis: a narrative review. Pulm Ther. 2022;8(1):43–55. https://doi.org/10.1007/s41030-022-00181-0.
Poola N, Due B, Wright D, Brooks LR, Zaman F. Pharmacokinetics and pharmacodynamics of repository corticotropin injection compared with synthetic ACTH1-24 depot and methylprednisolone in healthy subjects. Clin Pharmacol Drug Dev. 2022;11(4):502–15. https://doi.org/10.1002/cpdd.1020.
Huang YJ, Galen K, Zweifel B, Brooks LR, Wright AD. Distinct binding and signaling activity of Acthar Gel compared to other melanocortin receptor agonists. J Recept Signal Transduct Res. 2021;41(5):425–33. https://doi.org/10.1080/10799893.2020.1818094.
Benko AL, McAloose CA, Becker PM, Wright D, Sunyer T, Kawasawa YI, et al. Repository corticotrophin injection exerts direct acute effects on human B cell gene expression distinct from the actions of glucocorticoids. Clin Exp Immunol. 2018;192(1):68–81. https://doi.org/10.1111/cei.13089.
Healy LM, Jang JH, Lin YH, Rao V, Antel JP, D. W. Melanocortin receptor mediated anti-inflammatory effect of repository corticotropin injection on human monocyte derived macrophages. Mult Scler J. 2017;23 Suppl 3.
Benko AL, Wright AD, Sunyer T, Olsen NJ, Kovacs WJ. Individual pituitary neuropeptides do not recapitulate the effects of repository corticotropin (Acthar®) on human B cells in vitro. J Neuroimmunol. 2021;353: 577522. https://doi.org/10.1016/j.jneuroim.2021.577522.
Olsen NJ, Benko AL, McAloose CA, Becker PM, Wright D, Sunyer T, et al. Repository corticotropin injection reverses critical elements of the TLR9/B cell receptor activation response in human B cells in vitro. Clin Immunol. 2019;201:70–8. https://doi.org/10.1016/j.clim.2019.02.009.
Cusick MF, Libbey JE, Oh L, Jordan S, Fujinami RS. Acthar gel treatment suppresses acute exacerbations in a murine model of relapsing-remitting multiple sclerosis. Autoimmunity. 2015;48(4):222–30. https://doi.org/10.3109/08916934.2014.984836.
Approval Package for H.P. Acthar Gel; 2010. https://citronresearch.com/wp-content/uploads/2012/07/Questcor-022432Orig1_Original_Approval_Pkg.pdf. Accessed 1 Mar 2022.
Hollister LE, Page IH, Pfeiffer CC, Visscher MB. The Kefauver-Harris amendments of 1962: a critical appraisal of the first five years. J Clin Pharmacol J New Drugs. 1968;8(2):69–73. https://doi.org/10.1002/j.1552-4604.1968.tb00250.x.
Burnham TC, Huang S, Lo AW. Pricing for survival in the biopharma industry: a case study of Acthar Gel and Questcor Pharmaceuticals. J Invest Manag. 2017;15(4):69–91. https://doi.org/10.2139/ssrn.3040369.
Arnason BG, Berkovich R, Catania A, Lisak RP, Zaidi M. Mechanisms of action of adrenocorticotropic hormone and other melanocortins relevant to the clinical management of patients with multiple sclerosis. Mult Scler. 2013;19(2):130–6. https://doi.org/10.1177/1352458512458844.
Arrat H, Lukas TJ, Siddique T. ACTH (Acthar Gel) reduces toxic SOD1 protein linked to amyotrophic lateral sclerosis in transgenic mice: a novel observation. PLoS One. 2015;10(5): e0125638. https://doi.org/10.1371/journal.pone.0125638.
Berkovich R, Agius MA. Mechanisms of action of ACTH in the management of relapsing forms of multiple sclerosis. Ther Adv Neurol Disord. 2014;7(2):83–96. https://doi.org/10.1177/1756285613518599.
Decker DA, Grant C, Oh L, Becker PM, Young D, Jordan S. Immunomodulatory effects of H.P. Acthar Gel on B cell development in the NZB/W F1 mouse model of systemic lupus erythematosus. Lupus. 2014;23(8):802–12. https://doi.org/10.1177/0961203314531840.
Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97(3):375–9.
Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62(10):1668–81. https://doi.org/10.1212/01.wnl.0000127773.72699.c8.
Center for Drug Evaluation and Research. Action Memo for NDA 22-432, for the Use of H.P. Acthar Gel inthe Treatment of Infantile Spasms. Center for Drug Evaluation and Research; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022432Orig1s0900SumR.pdf. Accessed 2 Feb 2020.
Go CY, Mackay MT, Weiss SK, Stephens D, Adams-Webber T, Ashwal S, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78(24):1974–80. https://doi.org/10.1212/WNL.0b013e318259e2cf.
Mallinckrodt Pharmaceuticals. Acthar® Gel (repository corticotropin injection) reference bibliography; 2021. https://www.mallinckrodt.com/globalassets/documents/corporate/bibliography/acthar-gel-repository-corticotropin-injection-reference-bibliography.pdf. Accessed 21 Dec 2022.
Facts about Acthar® Gel; 2021. https://actharhcp.com/. Accessed 21 Dec 2022.
Fleischmann R, Furst DE. Safety of repository corticotropin injection as an adjunctive therapy for the treatment of rheumatoid arthritis. Expert Opin Drug Saf. 2020;19(8):935–44. https://doi.org/10.1080/14740338.2020.1779219.
Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327–44. https://doi.org/10.1007/s40744-020-00199-3.
Fleischmann R, Hayes K, Ahn SW, Wan GJ, Panaccio MP, Karlsson D, et al. Post hoc analysis of the correlation between patient-reported outcomes and clinical response to repository corticotropin injection for persistently active rheumatoid arthritis. Rheumatol Ther. 2022;9(2):435–46. https://doi.org/10.1007/s40744-021-00412-x.
Fleischmann R, Hayes K, Ahn SW, Wan GJ, Panaccio M, Karlsson D, et al. Post hoc analysis of predictors of clinical response to repository corticotropin injection in persistently active rheumatoid arthritis. Rheumatol Ther. 2022;9(2):649–61. https://doi.org/10.1007/s40744-022-00429-w.
Hayes K, Panaccio MP, Goel N, Fahim M. Patient characteristics and indicators of treatment initiation with repository corticotropin injection in patients with rheumatoid arthritis: a claims database analysis. Rheumatol Ther. 2021;8(1):327–46. https://doi.org/10.1007/s40744-020-00272-x.
Hayes K, Panaccio MP, Houston P, Niewoehner J, Fahim M, Wan GJ, et al. Real-world treatment patterns and outcomes from an electronic medical records database for patients with rheumatoid arthritis treated with repository corticotropin injection. Open Access Rheumatol. 2021;13:315–23. https://doi.org/10.2147/OARRR.S329766.
Busch H, Wan GJ, Niewoehner J, Houston P, Su Y, Clinton C, et al. Real-world treatment patterns for repository corticotropin injection in patients with rheumatoid arthritis. Drugs Context. 2022;11:1–12. https://doi.org/10.7573/dic.2021-10-4.
Fiechtner JJ, Montroy T. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: a single-site, open-label trial. Lupus. 2014;23(9):905–12. https://doi.org/10.1177/0961203314532562.
Fiechtner JJ, Montroy T. Six months’ treatment of moderately to severely active systemic lupus erythematosus with repository corticotropin injection: an extension of a single-site, open-label trial. J Immunol Clin Res. 2016;3(1):1025–30.
Furie R, Mitrane M, Zhao E, Das M, Li D, Becker PM. Efficacy and tolerability of repository corticotropin injection in patients with persistently active SLE: results of a phase 4, randomised, controlled pilot study. Lupus Sci Med. 2016;3(1): e000180. https://doi.org/10.1136/lupus-2016-000180.
Furie RA, Mitrane M, Zhao E, Becker PM. Repository corticotropin injection in patients with persistently active SLE requiring corticosteroids: post hoc analysis of results from a two-part, 52-week pilot study. Lupus Sci Med. 2017;4(1): e000240. https://doi.org/10.1136/lupus-2017-000240.
Askanase AD, Zhao E, Zhu J, Bilyk R, Furie RA. Repository corticotropin injection for persistently active systemic lupus erythematosus: results from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial. Rheumatol Ther. 2020;7(4):893–908. https://doi.org/10.1007/s40744-020-00236-1.
Askanase AD, Wan GJ, Panaccio MP, Zhao E, Zhu J, Bilyk R, et al. Patient-reported outcomes from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial of repository corticotropin injection (Acthar® Gel) for persistently active systemic lupus erythematosus. Rheumatol Ther. 2021;8(1):573–84. https://doi.org/10.1007/s40744-021-00294-z.
Askanase AD, Wright D, Zhao E, Zhu J, Bilyk R, Furie RA. Post hoc biomarker analyses from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial of repository corticotropin injection (Acthar® Gel) for persistently active systemic lupus erythematosus. Rheumatol Ther. 2021;8(4):1871–86. https://doi.org/10.1007/s40744-021-00351-7.
Askanase AD, Furie RA. A narrative review of repository corticotropin injection for the treatment of systemic lupus erythematosus. Adv Ther. 2022;39(7):3088–103. https://doi.org/10.1007/s12325-022-02160-y.
Levine T, Malone J, Efthimiou P, Tandan R, Dikranian A, Levine A, et al. H.P. Acthar® Gel in dermatomyositis and polymyositis treatment registry: an interim analysis. J Neurol Disord. 2016;4(5):2. https://doi.org/10.4172/2329-6895.1000292.
Fernandez A. Interim results of an open-label study assessing efficacy and safety of adrenocorticotropic hormone gel for treatment of refractory cutaneous manifestations of dermatomyositis [ACR Abstract]. Arthritis Rheum. 2018;70.
Aggarwal R, Marder G, Koontz DC, Nandkumar P, Qi Z, Oddis CV. Efficacy and safety of adrenocorticotropic hormone gel in refractory dermatomyositis and polymyositis. Ann Rheum Dis. 2018;77(5):720–7. https://doi.org/10.1136/annrheumdis-2017-212047.
Ho-Mahler N, Turner B, Eaddy M, Hanke ML, Nelson WW. Treatment with repository corticotropin injection in patients with rheumatoid arthritis, systemic lupus erythematosus, and dermatomyositis/polymyositis. Open Access Rheumatol. 2020;12:21–8. https://doi.org/10.2147/OARRR.S231667.
Kaplan J, Miller T, Baker M, Due B, Zhao E. A prospective observational registry of repository corticotropin injection (Acthar® Gel) for the treatment of multiple sclerosis relapse. Front Neurol. 2020;11: 598496. https://doi.org/10.3389/fneur.2020.598496.
Kaplan J, Miller T, Baker M, Due B, Zhao E. Repository corticotropin injection improves quality metrics in an observational study of multiple sclerosis relapse. Neurodegener Dis Manag. 2021;11(6):469–76. https://doi.org/10.2217/nmt-2021-0030.
Wynn D, Goldstick L, Bauer W, Zhao E, Tarau E, Cohen JA, et al. Results from a multicenter, randomized, double-blind, placebo-controlled study of repository corticotropin injection for multiple sclerosis relapse that did not adequately respond to corticosteroids. CNS Neurosci Ther. 2022;28(3):364–71. https://doi.org/10.1111/cns.13789.
Sharon Y, Chu DS. Adrenocorticotropic hormone analogue as novel treatment regimen in ocular cicatricial pemphigoid. Am J Ophthalmol Case Rep. 2018;10:264–7. https://doi.org/10.1016/j.ajoc.2018.03.018.
Scannell Bryan M, Sergott RC. Change in visual acuity and retinal structures following repository corticotropin injection (RCI) therapy in patients with acute demyelinating optic neuritis: Improvement in low contrast visual acuity in both affected and contralateral eyes in a single-armed open-label study. J Neurol Sci. 2019;407: 116505. https://doi.org/10.1016/j.jns.2019.116505.
Nelson WW, Lima AF, Kranyak J, Opong-Owusu B, Ciepielewska G, Gallagher JR, et al. Retrospective medical record review to describe use of repository corticotropin injection among patients with uveitis in the United States. J Ocul Pharmacol Ther. 2019;35(3):182–8. https://doi.org/10.1089/jop.2018.0090.
Anesi SD, Chang PY, Maleki A, Stephenson A, Montieth A, Filipowicz A, et al. Treatment of noninfectious retinal vasculitis using subcutaneous repository corticotropin injection. J Ophthalmic Vis Res. 2021;16(2):219–33. https://doi.org/10.18502/jovr.v16i2.9086.
Wirta D, McLaurin E, Ousler G, Liu J, Kacmaz RO, Grieco J. Repository corticotropin injection (Acthar® Gel) for refractory severe noninfectious keratitis: efficacy and safety from a phase 4, multicenter, open-label study. Ophthalmol Ther. 2021;10(4):1077–92. https://doi.org/10.1007/s40123-021-00400-y.
Toyos M, Toyos R, Jodoin B, Bunch R. Efficacy and safety of repository corticotropin injection for moderate and severe dry eye disease: a prospective, open-label, phase iv pilot study. Ophthalmol Ther. 2022;11(3):1231–40. https://doi.org/10.1007/s40123-022-00501-2.
Sharon Y, Anesi SD, Martinez CE, Huang AJW, Foster CS, Chu DS. Repository corticotropin injection as an alternative treatment for refractory ocular mucous membrane pemphigoid. Cornea. 2022;41(1):45–51. https://doi.org/10.1097/ICO.0000000000002771.
Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66–72. https://doi.org/10.1016/j.rmed.2015.11.007.
Baughman RP, Sweiss N, Keijsers R, Birring SS, Shipley R, Saketkoo LA, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313–22. https://doi.org/10.1007/s00408-017-9994-4.
Chopra I, Qin Y, Kranyak J, Gallagher JR, Heap K, Carroll S, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records. Ther Adv Respir Dis. 2019;13: 1753466619888127. https://doi.org/10.1177/1753466619888127.
Rahaghi FF, Sweiss NJ, Saketkoo LA, Scholand MB, Barney JB, Gerke AK, et al. Management of repository corticotrophin injection therapy for pulmonary sarcoidosis: a Delphi study. Eur Respir Rev. 2020;29(155): 190147. https://doi.org/10.1183/16000617.0147-2019.
Baughman RP, Mirsaeidi M, Zhao E, Houston P, Bilyk R. A phase 4, multicenter, randomized, double-blind, placebo-controlled exploratory study to assess the efficacy and safety of repository corticotropin injection in subjects with pulmonary sarcoidosis (PULSAR): study design and baseline characteristics. Chest. 2021;160(4):A1271–2. https://doi.org/10.1016/j.chest.2021.07.1159.
Mirsaeidi M, Baughman RP, Sahoo D, Tarau E. Results from a phase 4, multicenter, randomized, double-blind, placebo-controlled study of repository corticotropin injection for the treatment of pulmonary sarcoidosis. Pulm Ther. 2023;9(2):237–53. https://doi.org/10.1007/s41030-023-00222-2.
Hladunewich MA, Cattran D, Beck LH, Odutayo A, Sethi S, Ayalon R, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014;29(8):1570–7. https://doi.org/10.1093/ndt/gfu069.
Madan A, Mijovic-Das S, Stankovic A, Teehan G, Milward AS, Khastgir A. Acthar Gel in the treatment of nephrotic syndrome: a multicenter retrospective case series. BMC Nephrol. 2016;17:37. https://doi.org/10.1186/s12882-016-0241-7.
Filippone EJ, Dopson SJ, Rivers DM, Monk RD, Udani SM, Jafari G, et al. Adrenocorticotropic hormone analog use for podocytopathies. Int Med Case Rep J. 2016;9:125–33. https://doi.org/10.2147/IMCRJ.S104899.
Tumlin J, Galphin C, Santos R, Rovin B. Safety and efficacy of combination ACTHar Gel and tacrolimus in treatment-resistant focal segmental glomerulosclerosis and membranous glomerulopathy. Kidney Int Rep. 2017;2(5):924–32. https://doi.org/10.1016/j.ekir.2017.05.015.
Grafals M, Sharfuddin A. Adrenocorticotropic hormone in the treatment of focal segmental glomerulosclerosis following kidney transplantation. Transplant Proc. 2019;51(6):1831–7. https://doi.org/10.1016/j.transproceed.2019.04.052.
Zand L, Canetta P, Lafayette R, Aslam N, Jan N, Sethi S, et al. An open-label pilot study of adrenocorticotrophic hormone in the treatment of iga nephropathy at high risk of progression. Kidney Int Rep. 2020;5(1):58–65. https://doi.org/10.1016/j.ekir.2019.10.007.
Acknowledgements
Medical writing and editorial support were provided by MedLogix Communications, LLC, under the direction of the authors, and were funded by Mallinckrodt Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author Contributions
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and contributed to the conception or design of the work; acquisition, analysis, or interpretation of data; drafting or critically revising the work for important intellectual content; and final approval of the manuscript.
Funding
This review paper was funded by Mallinckrodt Pharmaceuticals.
Conflict of Interest
Anca Askanase has been an investigator or consultant for AbbVie, Amgen, AstraZeneca, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, Idorsia, Janssen, Genentech, GSK, Mallinckrodt, Pfizer, and UCB. Abdul Abdellatif has been a consultant and paid speaker for Horizon Therapeutics. David Chu has received consulting fees or a research grant from Aldeyra, Bausch and Lomb, Kriya, Mallinckrodt, and Santen. Dhiman Basu and Mehdi Mirsaeidi have no disclosures to report. Jeffrey Kaplan has received fees as a speaker or consultant for Alexion, Allergan, Amgen, Biogen, Biohaven, Bristol Myers Squibb, EMD Serono, Genentech, Genzyme, Horizon, Impel, Janssen, Lundbeck, Mallinckrodt, Merck, Novartis, and Teva.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Consent for Publication
Not applicable.
Data Availability
Data and study material are available on reasonable request from the corresponding author.
Code Availability
Not applicable.
Additional information
For a podcast discussion by the authors on Acthar® Gel see online [https://doi.org/10.1007/s40261-023-01303-5]
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kaplan, J., Askanase, A., Chu, D. et al. Acthar® Gel Treatment for Patients with Autoimmune and Inflammatory Diseases: An Historical Perspective and Characterization of Clinical Evidence. Clin Drug Investig 43, 739–761 (2023). https://doi.org/10.1007/s40261-023-01303-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-023-01303-5