Abstract
Background and Objective
Several studies on use of erenumab for migraine treatment have been published over recent years. However, its long-term safety and effectiveness have not been consistently established in the literature yet. We aimed to perform a qualitative and quantitative analysis of the long-term safety and effectiveness of erenumab for the treatment of migraine headaches.
Methods
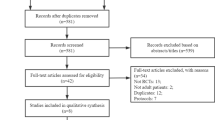
Long-term follow-up was defined as ≥ 1 year. PubMed, Embase and Cochrane Library were systematically searched from inception to 14 June 2022 for studies meeting the inclusion criteria. Risk of bias was assessed using the Newcastle-Ottawa Scale.
Results
Fourteen studies, comprising 3574 patients, were included. The total follow-up period ranged from 48 to 268 weeks (i.e., 1 year to 5.6 years). Pooled estimate rates for all adverse events (AEs) were 63% (95% CI 46–78); for serious AEs, 3% (95% CI 1–7); and for AEs leading to discontinuation of erenumab, 3% (95% CI 2–5). Reduction in monthly migraine days (MMDs) was −6.98 (95% CI −8.90 to −5.05) and in migraine-specific medication days (MSMDs) was − 6.09 (95% CI − 9.43 to − 2.75). More than half (57%; 95% CI 51–63) and around one-third (35%; 95% CI 28–42) of patients presented with reductions of ≥ 50% and ≥ 75% in MMDs, respectively. Headache Impact Test-6 (HIT-6) score was decreased by −9.68 points (95% CI − 12.03 to − 7.34). Nine studies were considered of poor methodological quality and five of fair quality.
Conclusions
Erenumab has a favorable safety profile, with a low incidence of serious AEs, and sustained efficacy over ≥1 year of follow-up in the treatment of migraine.







Similar content being viewed by others
References
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia Int J Headache. 2018 Jan;38(1):1–211.
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–22.
Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, on behalf of Lifting The Burden: the Global Campaign against Headache. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137.
Eigenbrodt AK, Ashina H, Khan S, Diener HC, Mitsikostas DD, Sinclair AJ, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. 2021;17(8):501–14.
Drellia K, Kokoti L, Deligianni CI, Papadopoulos D, Mitsikostas DD. Anti-CGRP monoclonal antibodies for migraine prevention: A systematic review and likelihood to help or harm analysis. Cephalalgia Int J Headache. 2021;41(7):851–64.
Sette L, Caponnetto V, Ornello R, Nežádal T, Čtrnáctá D, Šípková J, Matoušová Z, Sacco S. Acute medication use in patients with migraine treated with monoclonal antibodies acting on the CGRP pathway: results from a multicenter study and proposal of a new index. Front Neurol. 2022;28(13): 846717.
Bottiroli S, De Icco R, Vaghi G, Pazzi S, Guaschino E, Allena M, Ghiotto N, Martinelli D, Tassorelli C, Sances G. Psychological predictors of negative treatment outcome with Erenumab in chronic migraine: data from an open label long-term prospective study. J Headache Pain. 2021;22(1):114 (Erratum in: J Headache Pain. 2021 Nov 3;22(1):131).
Nowaczewska M, Straburzyński M, Waliszewska-Prosół M, Meder G, Janiak-Kiszka J, Kaźmierczak W. Cerebral blood flow and other predictors of responsiveness to erenumab and fremanezumab in migraine—a real-life study. Front Neurol. 2022;13: 895476.
Barbanti P, Aurilia C, Cevoli S, Egeo G, Fofi L, Messina R, et al. Long-term (48 weeks) effectiveness, safety, and tolerability of erenumab in the prevention of high-frequency episodic and chronic migraine in a real world: Results of the EARLY 2 study. Headache. 2021;61(9):1351–63.
Ornello R, Baraldi C, Guerzoni S, Lambru G, Andreou AP, Raffaelli B, Gendolla A, Barbanti P, Aurilia C, Egeo G, Cevoli S, Favoni V, Vernieri F, Altamura C, Russo A, Silvestro M, Valle ED, Mancioli A, Ranieri A, Alfieri G, Latysheva N, Filatova E, Talbot J, Cheng S, Holle D, Scheffler A, Nežádal T, Čtrnáctá D, Šípková J, Matoušová Z, Casalena A, Maddestra M, Viola S, Affaitati G, Giamberardino MA, Pistoia F, Reuter U, Sacco S. Comparing the relative and absolute effect of erenumab: is a 50% response enough? Results from the ESTEEMen study. J Headache Pain. 2022;23(1):38.
U.S. Food and Drug Administration (FDA). AIMOVIGTM (erenumab-aooe) injection, for subcutaneous use Initial U.S. Approval: 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf. Accessed 10 June 2022.
Cainazzo MM, Baraldi C, Ferrari A, Lo Castro F, Pani L, Guerzoni S. Erenumab for the preventive treatment of chronic migraine complicated with medication overuse headache: an observational, retrospective, 12-month real-life study. Neurol Sci. 2021;42(10):4193–202.
Nsaka M, Scheffler A, Wurthmann S, Schenk H, Kleinschnitz C, Glas M, Holle D. Real-world evidence following a mandatory treatment break after a 1-year prophylactic treatment with calcitonin gene-related peptide (pathway) monoclonal antibodies. Brain Behav. 2022;12(7): e2662.
Gantenbein AR, Agosti R, Gobbi C, Flügel D, Schankin CJ, Viceic D, et al. Impact on monthly migraine days of discontinuing anti-CGRP antibodies after one year of treatment—a real-life cohort study. Cephalalgia. 2021;41(11–12):1181–6.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane, 2022. www.training.cochrane.org/handbook. Assessed 8 June 2022.
Evans SR. Clinical trial structures. J Exp Stroke Transl Med. 2010;3(1):8–18.
Wells G, Shea B, O’Connell D, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute, 2021. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 June 2022.
Maraia Z, Ricci D, Rocchi MBL, Moretti A, Bufarini C, Cavaliere A, Peverini M. Real-life analysis with erenumab: first target therapy in the episodic and chronic migraine’s prophylaxis. J Clin Med. 2021;10(19):4425.
Pellitteri G, Pez S, Nilo A, Surcinelli A, Gigli GL, Lettieri C, et al. Erenumab impact on sleep assessed with questionnaires and home-polysomnography in patients with migraine: the ERESON Study. Front Neurol. 2022;13(13): 869677.
Ferrari MD, Reuter U, Goadsby PJ, Paiva da Silva Lima G, Mondal S, Wen S, et al. Two-year efficacy and safety of erenumab in participants with episodic migraine and 2–4 prior preventive treatment failures: results from the LIBERTY study. J Neurol Neurosurg Psychiatry. 2022;93(3):254–62.
Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick DW, Xue F, et al. Long-term efficacy and safety of erenumab in migraine prevention: results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021;28(5):1716–25.
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. One-year sustained efficacy of erenumab in episodic migraine: results of the STRIVE study. Neurology. 2020;95(5):e469–79.
Sakai F, Takeshima T, Tatsuoka Y, Hirata K, Cheng S, Numachi Y, et al. Long-term efficacy and safety during open-label erenumab treatment in Japanese patients with episodic migraine. Headache J Head Face Pain. 2021;61(4):653–61.
Tepper SJ, Ashina M, Reuter U, Brandes JL, Doležil D, Silberstein SD, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: Results from a 52-week, open-label extension study. Cephalalgia. 2020;40(6):543–53.
Cullum CK, Do TP, Ashina M, Bendtsen L, Hugger SS, Iljazi A, et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: a 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61.
Baraldi C, Castro FL, Cainazzo MM, Pani L, Guerzoni S. Predictors of response to erenumab after 12 months of treatment. Brain Behav. 2021;11(8): e2260.
Eghtesadi M, Leroux E, Pagé G. Real-life response to erenumab in a therapy-resistant case series of migraine patients from the Province of Québec, Eastern Canada. Clin Drug Investig. 2021;41(8):733–9.
Schoenen J, Timmermans G, Nonis R, Manise M, Fumal A, Gérard P. Erenumab for migraine prevention in a 1-year compassionate use program: efficacy, tolerability, and differences between clinical phenotypes. Front Neurol. 2021;10(12): 805334.
Hirata K, Sakai F, Takeshima T, Imai N, Matsumori Y, Yoshida R, et al. Efficacy and safety of erenumab in Japanese migraine patients with prior preventive treatment failure or concomitant preventive treatment: subgroup analyses of a phase 3, randomized trial. J Headache Pain. 2021;22(1):110.
Ornello R, Casalena A, Frattale I, Gabriele A, Affaitati G, Giamberardino MA, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21(1):32.
Becker WJ, Spacey S, Leroux E, Giammarco R, Gladstone J, Christie S, et al. A real-world, observational study of erenumab for migraine prevention in Canadian patients. Headache J Head Face Pain. 2022;62(4):522–9.
Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: a Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–37.
Schenk H, Holle D, Nsaka M, Kleinschnitz C, Glas M, Scheffler A. Twelve-month safety, tolerability and susceptibility to adverse events of prophylactic migraine therapy with erenumab: a retrospective real-world study. J Headache Pain. 2022;23(1):55.
Lattanzi S, Brigo F, Trinka E, Vernieri F, Corradetti T, Dobran M, et al. Erenumab for Preventive Treatment of Migraine: A Systematic Review and Meta-Analysis of Efficacy and Safety. Drugs. 2019;79(4):417–31.
Zhu C, Guan J, Xiao H, Luo W, Tong R. Erenumab safety and efficacy in migraine. Medicine (Baltimore). 2019;98(52): e18483.
Wang X, He Q, Wen D, Ma L, You C. Efficacy and safety of erenumab in migraine prevention: evidences from direct and indirect comparisons. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2022;43(4):2751–8.
Deng H, Li GG, Nie H, Feng YY, Guo GY, Guo WL, et al. Efficacy and safety of calcitonin-gene-related peptide binding monoclonal antibodies for the preventive treatment of episodic migraine—an updated systematic review and meta-analysis. BMC Neurol. 2020;20(1):57.
Masoud AT, Hasan MT, Sayed A, Edward HN, Amer AM, Naga AE, et al. Efficacy of calcitonin gene-related peptide (CGRP) receptor blockers in reducing the number of monthly migraine headache days (MHDs): a network meta-analysis of randomized controlled trials. J Neurol Sci. 2021;15(427): 117505.
Soni P, Chawla E. Efficacy and safety of anti-calcitonin gene-related peptide monoclonal antibodies for treatment of chronic migraine: a systematic review and network meta-analysis. Clin Neurol Neurosurg. 2021;209: 106893.
Wang X, Chen Y, Song J, You C. Efficacy and Safety of Monoclonal Antibody Against Calcitonin Gene-Related Peptide or Its Receptor for Migraine: A Systematic Review and Network Meta-analysis. Front Pharmacol. 2021;12: 649143.
Xu D, Chen D, Zhu LN, Tan G, Wang HJ, Zhang Y, et al. Safety and tolerability of calcitonin-gene-related peptide binding monoclonal antibodies for the prevention of episodic migraine—a meta-analysis of randomized controlled trials. Cephalalgia Int J Headache. 2019;39(9):1164–79.
Zhu Y, Liu Y, Zhao J, Han Q, Liu L, Shen X. The efficacy and safety of calcitonin gene-related peptide monoclonal antibody for episodic migraine: a meta-analysis. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2018;39(12):2097–106.
Alasad YW, Asha MZ. Monoclonal antibodies as a preventive therapy for migraine: a meta-analysis. Clin Neurol Neurosurg. 2020;195: 105900.
Ashina M, Kudrow D, Reuter U, Dolezil D, Silberstein S, Tepper SJ, et al. Long-term tolerability and nonvascular safety of erenumab, a novel calcitonin gene-related peptide receptor antagonist for prevention of migraine: a pooled analysis of four placebo-controlled trials with long-term extensions. Cephalalgia. 2019;39(14):1798–808.
Ashina M, Reuter U, Dodick D, Zhang F, Ritter S, Stites T, Paiva da Silva Lima G, Arkuszewski M, Goadsby P. Long-term safety and tolerability of erenumab in episodic migraine: a pooled analysis from two clinical trials and their extension phases (P3-2.005). Neurology. 2022;98(18 Supplement):324.
Zhou Y, Zhang F, Starcevic Manning M, Hu Z, Hsu CP, Chen PW, Peng C, Loop B, Mytych DT, Paiva ds Silva Lima G. Immunogenicity of erenumab: a pooled analysis of six placebo-controlled trials with long-term extensions. Cephalalgia. 2022;42(8):749–60.
Pellesi L, De Icco R, Alawie HY, Andersen M, Liang D, Amirguliyev S, et al. A systematic review, meta-analysis and meta-regression evaluating the adverse reactions to erenumab in the preventive treatment of migraine. Expert Opin Drug Saf. 2021;20(4):467–74.
Tepper SJ, Diener HC, Ashina M, Brandes JL, Friedman DI, Reuter U, et al. Erenumab in chronic migraine with medication overuse: Subgroup analysis of a randomized trial. Neurology. 2019;92(20):e2309–20.
Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6TM) across episodic and chronic migraine. Cephalalgia. 2011;31(3):357–67.
Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71(8):559–66.
Pavlović JM. The impact of midlife on migraine in women: summary of current views. Womens Midlife Health. 2020;6(6):11.
Lampl C, Kraus V, Lehner K, Loop B, Chehrenama M, Maczynska Z, Ritter S, Klatt J, Snellman J. Safety and tolerability of erenumab in individuals with episodic or chronic migraine across age groups: a pooled analysis of placebo-controlled trials. J Headache Pain. 2022;23(1):104.
de Vries LS, van der Arend BWH, Maassen VanDenBrink A, Terwindt GM. Blood pressure in patients with migraine treated with monoclonal anti-CGRP (Receptor) antibodies: a prospective follow-up study. Neurology. 2022;99(17):e1897–904.
Gérard AO, Merino D, Van Obberghen EK, Rocher F, Destere A, Lantéri-Minet M, Drici MD. Calcitonin gene-related peptide-targeting drugs and Raynaud’s phenomenon: a real-world potential safety signal from the WHO pharmacovigilance database. J Headache Pain. 2022;23(1):53.
Joshi N, McAree M, Klimowich K, Cahill K, Janora D. Oral candidiasis in a migraine patient taking erenumab and galcanezumab: a case report. Compr Clin Med. 2020;2(5):658–61.
Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382–90.
Vernieri F, Brunelli N, Messina R, Costa CM, Colombo B, Torelli P, et al. Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study. J Headache Pain. 2021;22(1):154.
De Matteis E, Affaitati G, Frattale I, Caponnetto V, Pistoia F, Giamberardino MA, et al. Early outcomes of migraine after erenumab discontinuation: data from a real-life setting. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2021;42(8):3297–303.
Guerzoni S, Baraldi C, Pensato U, Favoni V, Lo Castro F, Cainazzo MM, et al. Chronic migraine evolution after 3 months from erenumab suspension: real-world-evidence-life data. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2022;43(6):3823–30.
Schiano di Cola F, Caratozzolo S, Venturelli E, Balducci U, Sidoti V, Pari E, et al. Erenumab discontinuation after 12-month treatment: a multicentric, observational real-life study. Neurol Clin Pract. 2021;11(6):e834–9.
Terhart M, Mecklenburg J, Neeb L, Overeem LH, Siebert A, Steinicke M, Raffaelli B, Reuter U. Deterioration of headache impact and health-related quality of life in migraine patients after cessation of preventive treatment with CGRP(-receptor) antibodies. J Headache Pain. 2021;22(1):158.
Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Stat Med. 2002;21(11):1503–11.
Day RO, Williams KM. Open-label extension studies: do they provide meaningful information on the safety of new drugs? Drug Saf. 2007;30(2):93–105.
de Lusignan S, Crawford L, Munro N. Creating and using real-world evidence to answer questions about clinical effectiveness. J Innov Health Inform. 2015;22(3):368–73.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethics Approval
No ethical approval was required because this study synthesized and analyzed data from already published studies, in which informed consent was obtained by primary investigators.
Data Availability
All data generated or analyzed during this study are included in this published article (and its electronic supplemental material).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Authors' Contributions
FFB: study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript and critical revision. JPMT: analysis and interpretation of results and critical revision. RBR: acquisition of data and critical revision. GIC: draft manuscript preparation. GBN: draft manuscript preparation. All authors have read and agreed to the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bomtempo, F.F., Rocha, R.B., Cenci, G.I. et al. Long-Term Safety and Effectiveness of Erenumab in Patients with Migraine: A Systematic Review and Single-Arm Meta-analysis. Clin Drug Investig 43, 45–59 (2023). https://doi.org/10.1007/s40261-022-01230-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01230-x