Abstract
Background and Objective
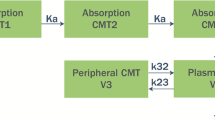
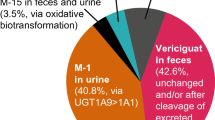
Vixotrigine is a voltage-dependent and use-dependent sodium channel blocker in development for the treatment of neuropathic pain. Metabolism of vixotrigine is primarily through glucuronidation, resulting in the major M13 metabolite. Two additional major metabolites formed are M14 and M16. This study was designed to evaluate the effects of a uridine diphosphate-glucuronosyltransferase inhibitor, valproic acid, on vixotrigine pharmacokinetics.
Methods
This open-label, fixed-sequence, phase I study enrolled 30 healthy volunteers who received a single dose of vixotrigine 150 mg on day 1 and day 16 following an 8-h fast. On days 8–22, volunteers received valproic acid 500 mg three times daily. A mixed-effects model was used to analyze the effect of valproic acid on the natural log-transformed pharmacokinetic parameters of vixotrigine and its metabolites including maximum concentration and area under the concentration–time curve from time zero to infinity.
Results
Vixotrigine systemic exposure (area under the concentration–time curve from time zero to infinity) was increased by approximately 70% following the addition of valproic acid with a negligible effect on maximum concentration. Valproic acid administration also impacted vixotrigine metabolites: M13 exposure decreased by approximately 50% and M13 maximum concentration decreased by approximately 70%; increased exposure was noted for the M14 (approximately 100%) and M16 (approximately 70%) metabolites.
Conclusions
Valproic acid, a uridine diphosphate-glucuronosyltransferase inhibitor, significantly increased vixotrigine systemic exposure.
Clinical Trial Registration
ClinicalTrials.gov Identifier: NCT03385525.



Similar content being viewed by others
References
Macone A, Otis JAD. Neuropathic pain. Semin Neurol. 2018;38(6):644–53. https://doi.org/10.1055/s-0038-1673679.
Zilliox LA. Neuropathic pain. Continuum (Minneap Minn). 2017;23(2, Selected Topics in Outpatient Neurology):512–32. https://doi.org/10.1212/CON.0000000000000462.
Attal N. Pharmacological treatments of neuropathic pain: the latest recommendations. Rev Neurol (Paris). 2019;175(1–2):46–50. https://doi.org/10.1016/j.neurol.2018.08.005.
Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD. The role of voltage-gated sodium channels in pain signaling. Physiol Rev. 2019;99(2):1079–151. https://doi.org/10.1152/physrev.00052.2017.
Zakrzewska JM, Palmer J, Morisset V, Giblin GM, Obermann M, Ettlin DA, et al. Study Investigators. Safety and efficacy of a Nav17 selective sodium channel blocker in patients with trigeminal neuralgia: a double-blind, placebo-controlled, randomised withdrawal phase 2a trial. Lancet Neurol. 2017;16(4):291–300. https://doi.org/10.1016/S1474-4422(17)30005-4.
Naik H, Steiner DJ, Versavel M, Palmer J, Fong R. Safety, tolerability and pharmacokinetics of single and repeat doses of vixotrigine in healthy volunteers. Clin Transl Sci. 2021;14(4):1272–9. https://doi.org/10.1111/cts.12935.
Naik H, Zhao Y, Forrestal F, Cleall S, Bockbrader H, Chapel S. Population pharmacokinetics of vixotrigine in healthy volunteers and subjects with trigeminal neuralgia, painful lumbosacral radiculopathy and erythromelalgia. Eur J Drug Metab Pharmacokinet. 2021;46(3):395–404. https://doi.org/10.1007/s13318-021-00678-0.
Woodward C, Naik H, Versavel M, et al. Phase 1 study to evaluate the absorption, metabolism and excretion of the NAV1.7-selective sodium channel blocker BIIB074 [poster 162]. Presented at the International Society for the Study of Xenobiotics (ISSX) 21st North American Meeting; 24–28 September, 2017; Providence (RI).
Dunbar J, Versavel M, Zhao Y, Tate S, Morisset V, Giblin GMP, et al. Evaluation of the pharmacokinetic interaction between the voltage- and use-dependent Nav1.7 channel blocker vixotrigine and carbamazepine in healthy volunteers. Clin Pharmacol Drug Dev. 2020;9(1):62–73. https://doi.org/10.1002/cpdd.739.
Gugler R, von Unruh GE. Clinical pharmacokinetics of valproic acid. Clin Pharmacokinet. 1980;5(1):67–83. https://doi.org/10.2165/00003088-198005010-00002.
Ghodke-Puranik Y, Thorn CF, Lamba JK, Leeder JS, Song W, Birnbaum AK, et al. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2013;23(4):236–41. https://doi.org/10.1097/FPC.0b013e32835ea0b2.
Anderson GD, Yau MK, Gidal BE, Harris SJ, Levy RH, Lai AA, et al. Bidirectional interaction of valproate and lamotrigine in healthy subjects. Clin Pharmacol Ther. 1996;60(2):145–56. https://doi.org/10.1016/S0009-9236(96)90130-7.
Perucca E, Hebdige S, Frigo GM, Gatti G, Lecchini S, Crema A. Interaction between phenytoin and valproic acid: plasma protein binding and metabolic effects. Clin Pharmacol Ther. 1980;28(6):779–89. https://doi.org/10.1038/clpt.1980.235.
Lertora JJ, Rege AB, Greenspan DL, Akula S, George WJ, Hyslop NE Jr, et al. Pharmacokinetic interaction between zidovudine and valproic acid in patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1994;56(3):272–8. https://doi.org/10.1038/clpt.1994.137.
US Food and Drug Administration. FDA draft guidance 2012. Drug interaction studies-study design, data analysis, implications for dosing, and labeling recommendations. Silver Spring, MD: FDA.
US Department of Health and Human Services, US Food and Drug Administration. Guidance for industry: statistical approaches to establishing bioequivalence. 2001. Rockville, MD: FDA.
Ethell BT, Anderson GD, Burchell B. The effect of valproic acid on drug and steroid glucuronidation by expressed human UDP-glucuronosyltransferases. Biochem Pharmacol. 2003;65(9):1441–9. https://doi.org/10.1016/s0006-2952(03)00076-5.
Zaccara G, Perucca E. Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord. 2014;16(4):409–31. https://doi.org/10.1684/epd.2014.0714.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The clinical study was funded by Biogen.
Conflict of interest
YZ, MK, HF, MS, and HN: present or prior employees of and own stock/stock options in Biogen.
Ethics approval
The trial was registered at ClinicalTrials.gov (NCT03385525) and conducted in accordance with the Declaration of Helsinki principles and Good Clinical Practice guidelines. The institutional review board of the clinical site (Covance Clinical Research Unit, Alice, TX, USA) provided approval of the protocol.
Consent to participate
All participants provided informed written consent.
Consent for publication
Not applicable.
Availability of data and material
The data that support this clinical study are available upon reasonable request.
Code availability
Not applicable.
Author contributions
YZ and HN led the study design, data interpretation, and manuscript preparation. All authors contributed to the study execution and data analysis, and reviewed and provided input on the manuscript.
Additional information
Yuan Zhao and Himanshu Maik were prior employees of Biogen, Cambridge, MA, USA.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Kotecha, M., Finnigan, H. et al. Evaluation of the Effect of Uridine Diphosphate-Glucuronosyltransferases (UGT) Inhibition by Valproic Acid on Vixotrigine Pharmacokinetics in Healthy Volunteers. Clin Drug Investig 42, 829–837 (2022). https://doi.org/10.1007/s40261-022-01194-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01194-y