Abstract
Background and Objective
Peroxisome proliferator-activated receptors (PPARs) have recently become a focus of interest for their important roles in glucose and lipid metabolism. In humans, PPARα activation causes a decrease in plasma triglyceride (TG) levels, enhancement of high-density lipoprotein cholesterol (HDL-C) and simultaneous enhancement of very-low-density lipoprotein (VLDL) lipolysis, whereas PPARγ agonists act as insulin sensitizers and improve insulin resistance, which is very useful in patients with type 2 diabetes mellitus (T2DM). Saroglitazar magnesium is a dual PPAR agonist with potent predominant PPARα and moderate PPARγ activity and the first glitazar to be granted marketing authorization in India. This study was conducted to evaluate the oral bioavailability and safety and tolerability of a Lipaglyn™ (saroglitazar magnesium) 4-mg tablet in healthy, adult human subjects under fed relative to fasting conditions.
Methods
This was a single-dose, open-label, randomized, single-treatment, two-period, two-conditions (fed vs. fasting), two-sequence, crossover study planned in 54 healthy subjects. Food effect (high-calorie and high-fat breakfast) was examined by comparing pharmacokinetic data of saroglitazar and its metabolite saroglitazar sulfoxide in plasma samples collected pre-dose and serially up to 72 h post-dose. Pharmacokinetic data were analyzed using the standard non-compartmental approach.
Results
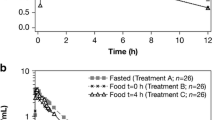
A total of 54 subjects were enrolled in the study, out of them 50 subjects had completed the study and were analyzed. The presence of food had a minor impact on the disposition of saroglitazar. While food reduced C max (maximum concentration) of saroglitazar by 30%, the extent of absorption as measured by AUC∞ (area under the concentration time curve from time zero to infinity) was not influenced. This was further supported by the bioequivalence data between fasted and fed conditions for saroglitazar, where 90% CIs (confidence intervals) of the adjusted geometric mean of the fed relative to the fasted condition ranged from 101.37% to 108.07% for AUC∞ and from 63.45% to 74.68% for C max. Other parameters such as T max (time of maximum concentration) and T 1/2 (elimination half-life) were not influenced by the food intake. Saroglitazar was well tolerated in the study, and the reported adverse events were mild in nature.
Conclusion
For the single-dose study, the absorption rate is affected by food as the 90% CI of C max is outside 80.00–125.00%. However, there is no impact of food on the extent of absorption of saroglitazar. The observed lower C max of saroglitazar with food has no clinical relevance since the therapeutic efficacy of saroglitazar was achieved after multiple-dose administration, suggesting the importance of total exposure.



Similar content being viewed by others
References
Morrish N, Wang S, Stevens L, Fuller J, Keen H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(S2):S14–21. doi:10.1007/pl00002934.
Diabetes. World Health Organization. 2017. Available at: http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed 20 July 2017.
Sever P, Dahlof B, Poulter N. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. ACC Curr J Rev. 2003;12(4):36. doi:10.1016/s1062-1458(03)00278-2.
Pedersen T. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Atheroscler Suppl. 2004;5(3):81–7. doi:10.1016/j.atherosclerosissup.2004.08.027.
Fruchart J, Sacks F, Hermans M, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patients. Diabetes Vasc Disease Res. 2008;5(4):319–35. doi:10.3132/dvdr.2008.046.
Dluhy R, McMahon G. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358(24):2630–3. doi:10.1056/nejme0804182.
Zoungas S, de Galan B, Ninomiya T, et al. Combined Effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care. 2009;32(11):2068–74. doi:10.2337/dc09-0959.
Del Pilar Solano M, Goldberg R. Management of diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2005;34(1):1–25. doi:10.1016/j.ecl.2005.01.001.
Dyslipidemia Management in Adults With Diabetes. 2017. http://care.diabetesjournals.org/content/27/suppl_1/s68. Accessed 20 July 2017.
Berger J, Moller D. The mechanisms of action of PPARs. Annu Rev Med. 2002;53(1):409–35. doi:10.1146/annurev.med.53.082901.104018.
Boitier E, Gautier J, Roberts R. Advances in understanding the regulation of apoptosis and mitosis by peroxisome-proliferator activated receptors in pre-clinical models: relevance for human health and disease-PubMed-NCBI. Ncbinlmnihgov. 2003. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC151270/. Accessed 20 July 2017.
Pascual G, Fong A, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature. 2005;437(7059):759–63. doi:10.1038/nature03988.
Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart J. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–93. doi:10.1161/01.cir.98.19.2088.
Gross B, Staels B. PPAR agonists: multimodal drugs for the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab. 2007;21(4):687–710. doi:10.1016/j.beem.2007.09.004.
Jani R, Kansagra K, Jain M, Patel H. Pharmacokinetics, Safety, and Tolerability of Saroglitazar (ZYH1), a predominantly PPARα agonist with moderate PPARγ agonist activity in healthy human subjects. Clinical Drug Investig. 2013;33(11):809–16. doi:10.1007/s40261-013-0128-3.
Rubenstrunk A, Hanf R, Hum D, Fruchart J, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids. 2007;1771(8):1065–81. doi:10.1016/j.bbalip.2007.02.003.
Aggarwal A. Saroglitazar: India’s answer to diabetic dyslipidemia. Int J Pharmacol Clin Sci. 2014;3(1):7–14.
Guidance for Industry Bioanalytical Method Validation. 2017https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf. Accessed 29 July 2017.
Wu P, Peters J, Harris R. adaptive increase in pyruvate dehydrogenase kinase 4 during starvation is mediated by peroxisome proliferator-activated receptor α. Biochem Biophys Res Commun. 2001;287(2):391–6. doi:10.1006/bbrc.2001.5608.
Lalloyer F, Vandewalle B, Percevault F, et al. peroxisome proliferator-activated receptor improves pancreatic adaptation to insulin resistance in obese mice and reduces lipotoxicity in human islets. Diabetes. 2006;55(6):1605–13. doi:10.2337/db06-0016.
Ravnskjaer K, Boergesen M, Rubi B, et al. Peroxisome Proliferator-activated receptor α (PPARα) potentiates, whereas PPARγ attenuates, glucose-stimulated insulin secretion in pancreatic β-Cells. Endocrinology. 2005;146(8):3266–76. doi:10.1210/en.2004-1430.
Staels B, Fruchart J. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54(8):2460–70. doi:10.2337/diabetes.54.8.2460.
Aravind Sosale S, Saboo B, Sosale B. Saroglitazar for the treatment of hypertriglyceridemia in patients with type 2 diabetes: current evidence. Diabetes Metab Syndr Obes Targets Ther. 2015;8:189. doi:10.2147/dmso.s49592.
Ruiz-Garcia A, Plotka A, O’Gorman M, Wang DD. Effect of food on the bioavailability of palbociclib. Cancer Chemother Pharmacol. 2017;79(3):527–33. doi:10.1007/s00280-017-3246-4.
Joh DA, Parikh N, Khurana V, Cognata Smith C, Vetticaden S. Effect of food on the pharmacokinetics of dronabinol oral solution versus dronabinol capsules in healthy volunteers. Clin Pharmacol. 2017;9:9–17. doi:10.2147/CPAA.S119676 (eCollection 2017).
Koskimies P, Katila K, Lammintausta R, Aaltonen AM, Vuorinen J, Saarni O, Scheinin M. Oral bioavailability of ospemifene improves with food intake. Int J Clin Pharmacol Ther. 2013;51(10):787–94. doi:10.5414/CP201873.
Flanagan SD, Bien PA, Muñoz KA, Minassian SL, Prokocimer PG. Pharmacokinetics of tedizolid following oral administration: single and multiple dose, effect of food, and comparison of two solid forms of the prodrug. Pharmacotherapy. 2014;34(3):240–50. doi:10.1002/phar.1337 Epub 2013 Aug 7.
Saleh S, Frey R, Becker C, Unger S, Wensing G, Mück W. Bioavailability, pharmacokinetics, and safety of riociguat given as an oral suspension or crushed tablet with and without food. Pulm Circ. 2016;6(Suppl 1):S66–74. doi:10.1086/685020.
Shapiro GI, Frank R, Dandamudi UB, Hengelage T, Zhao L, Gazi L, Porro MG, Woo MM, Lewis LD. The effect of food on the bioavailability of panobinostat, an orally active pan-histone deacetylase inhibitor, in patients with advanced cancer. Cancer Chemother Pharmacol. 2012;69(2):555–62. doi:10.1007/s00280-011-1758-x Epub 2011 Nov 6.
Hauns B, Hermann R, Hünnemeyer A, Herzog R, Hauschke D, Zech K, Bethke TD. Investigation of a potential food effect on the pharmacokinetics of roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, in healthy subjects. J Clin Pharmacol. 2006;46(10):1146–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict Interest
Maulik R. Patel, Kevinkumar A. Kansagra, Devang P. Parikh, Deven V. Parmar, Hardik B. Patel, Harilal V. Patel, Krupi V. Parmar, and Nuggehally R. Srinivas are employees of Zydus Research Centre and Mayur M. Soni, Uday S. Patil, Jaimik A. Patel, Swagat S. Gujarathi are employees of Cliantha Research Limited.
Funding
Cliantha Research Limited received funding from Cadila Healthcare Ltd. to plan, conduct, record, and report this study.
Ethical Approval
All of the study-related procedures were performed after gaining approval from the Independent Ethics Committee Aditya, Ahmedabad and in accordance with the ethical principles stipulated in the Declaration of Helsinki, ICMR ethical guidelines, International Conference on Harmonization (ICH) (Step 5) ‘Guidance on Good Clinical Practice’ (E6), Schedule Y of Drugs and Cosmetics Act, 2005.
Informed Consent
Written informed consent was obtained from all individual participants before enrollment into the study.
Rights and permissions
About this article
Cite this article
Patel, M.R., Kansagra, K.A., Parikh, D.P. et al. Effect of Food on the Pharmacokinetics of Saroglitazar Magnesium, a Novel Dual PPARαγ Agonist, in Healthy Adult Subjects. Clin Drug Investig 38, 57–65 (2018). https://doi.org/10.1007/s40261-017-0584-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-017-0584-2