Abstract
Background and Objective
Nucleos(t)ide analogue (NA) monotherapies are typically used as the primary treatment for chronic hepatitis B (CHB) patients, including lamivudine (LAM), telbivudine (TBV), adefovir (ADV), entecavir (ETV) and tenofovir (TDF). For high-resistance NAs (LAM, TBV, ADV), they can generate excellent clinical outcomes by using response-guided therapy; however, their pharmacoeconomic profiles remain unclear in China. We aimed to evaluate the cost effectiveness between response-guided therapies and monotherapies of NAs for Chinese hepatitis B e-antigen (HBeAg)-positive and -negative CHB patients.
Methods
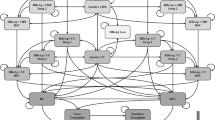
We constructed a Markov model to simulate CHB progression associated with 12 treatment strategies using effectiveness and cost data from the published literature. We measured the lifetime costs, quality adjusted life-years (QALYs) and incremental cost-effectiveness ratios (ICERs). One-way sensitivity (especially to extend the range of the TDF price) and probabilistic sensitivity analyses were used to explore the uncertainties of the model.
Results
For both HBeAg-positive and -negative patients, no treatment strategy generated the lowest lifetime costs (US$31,185–US$31,338) and QALYs (7.54–7.58). ETV and TDF monotherapies were not dominated by other treatments, whereas, the ICER of ETV monotherapy was the lowest (US$6112/QALY–US$8533/QALY). For each high-resistance NA, compared with its monotherapy, the ICERs of its response-guided therapies were below the willingness-to-pay threshold of US$22,833/QALY. Additionally, TDF monotherapy was the preferred treatment when its price dropped to US$1820/year or lower.
Conclusion
Among 12 treatment strategies evaluated, ETV monotherapy is the most cost-effective treatment for treatment-naive CHB patients in China. The response-guided therapies of high-resistance NAs are more cost-effective than their monotherapies.





Similar content being viewed by others
References
World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis b infection. Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ Accessed Jun 2016.
Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Hou JL, Lai W. The guideline of prevention and treatment for chronic hepatitis B: a 2015 update. Chin J Hepatol. 2015;23(12):888–905.
Chen CJ, Yang HI, Iloeje UH, REVEAL-HBV Study Group. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49(5 Suppl):S72–84.
Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–83.
Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Yu R, Fan R, Hou J. Chronic hepatitis B virus infection: epidemiology, prevention, and treatment in China. Front Med. 2014;8(2):135–44.
Keeffe EB, Zeuzem S, Koff RS, et al. Report of an international workshop: roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5(8):890–7.
Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357(25):2576–88.
Lu H, da Geng Y, Shen F, et al. Optimization of adefovir therapy in chronic hepatitis B according to baseline predictors and on-treatment HBV DNA: a 5-year prospective study. Virol J. 2011;8:444.
Zeuzem S, Gane E, Liaw YF, et al. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009;51(1):11–20.
Sun J, Xie Q, Tan D, et al. The 104-week efficacy and safety of telbivudine-based optimization strategy in chronic hepatitis B patients: a randomized, controlled study. Hepatology. 2014;59(4):1283–92.
Liang X, Cheng J, Sun Y, et al. Randomized, three-arm study to optimize lamivudine efficacy in hepatitis B e antigen-positive chronic hepatitis B patients. J Gastroenterol Hepatol. 2015;30(4):748–55.
Ma XJ, Chen XP, Chen XF, et al. Efficacy of lamivudine and adefovir de novo combination therapy or after mono-therapy in chronic hepatitis B patients. Chin J Hepatol. 2012;20(2):98–102.
Zhang C, Ke W, Gao Y, et al. Cost-effectiveness analysis of antiviral therapies for hepatitis B e antigen-positive chronic hepatitis B patients in China. Clin Drug Investig. 2015;35(3):197–209.
Zhang C, Ke W, Liu L, et al. Cost-effectiveness comparison of lamivudine plus adefovir combination treatment and nucleos(t)ide analog monotherapies in Chinese chronic hepatitis B patients. Drug Des Devel Ther. 2016;10:897–910.
Ke W, Zhang C, Liu L, et al. Cost-effectiveness analysis of tenofovir disoproxil fumarate for treatment of chronic hepatitis B in China. Hepatol Int. 2016;10(6):924–36.
Wu B, Li T, Chen H, Shen J. Cost-effectiveness of nucleoside analog therapy for hepatitis B in China: a Markov analysis. Value Health. 2010;13(5):592–600.
Lo AO, Wong VW, Wong GL, et al. Cost effectiveness of response-guided therapy with peginterferon in the treatment of chronic hepatitis B. Clin Gastroenterol Hepatol. 2015;13(2):377–385e5.
Wu G, Zhou W, Zhao Y, et al. Study on the natural history of chronic hepatitis B. Chin J Hepatol. 2002;10(1):46–8.
Huang H, Zhu CW, Yu XF, et al. The development of cirrhosis in Chinese patients with chronic hepaitits B. Chin Hepatol. 2007;6:437–40.
Xu B, Hu DC, Rosenberg DM, et al. Chronic hepatitis B: a long-term retrospective cohort study of disease progression in Shanghai, China. J Gastroenterol Hepatol. 2003;18(12):1345–52.
Tabulation on the 2010 Population Census of the People’s Republic of China. Available from: http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm. Accessed Sep 2015.
Chen YC, Chu CM, Yeh CT, Liaw YF. Natural course following the onset of cirrhosis in patients with chronic hepatitis B: a long-term follow-up study. Hepatol Int. 2007;1(1):267–73.
Hui AY, Chan HL, Leung NW, et al. Survival and prognostic indicators in patients with hepatitis B virus-related cirrhosis after onset of hepatic decompensation. J Clin Gastroenterol. 2002;34(5):569–72.
Wang SB, Wang JH, Chen J, et al. Natural history of liver cirrhosis in south China based on a large cohort study in one center: a follow-up study for up to 5 years in 920 patients. Chin Med (Engl). 2012;125(12):2157–62.
Lo CM, Fan ST, Liu CL, et al. The role and limitation of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2004;10(3):440–7.
Wu TJ, Chan KM, Chou HS, et al. Liver transplantation in patients with hepatitis B virus-related hepatocellular carcinoma: the influence of viral characteristics on clinical outcome. Ann Surg Oncol. 2013;20(11):3582–90.
Ha M, Zhang G, Diao S, et al. Rescue therapy for lamivudine-resistant chronic hepatitis B: adefovir monotherapy, adefovir plus lamivudine or entecavir combination therapy. Intern Med. 2012;51(12):1509–15.
Park MS, Kim BK, Kim KS, et al. Antiviral efficacies of currently available rescue therapies for multidrug-resistant chronic hepatitis B. Clin Mol Hepatol. 2013;19(1):29–35.
Lee YB, Lee JH, Choi WM, et al. Efficacy of adefovir-based combination therapy for patients with lamivudine- and entecavir-resistant chronic hepatitis B virus infection. Antimicrob Agents Chemother. 2013;57(12):6325–32.
Kang SH, Yim HJ, Kim HR, et al. Comparison of lamivudine plus adefovir therapy versus entecavir with or without adefovir therapy for adefovir-resistant chronic hepatitis B. J Clin Gastroenterol. 2014;48(10):889–95.
Jia JD, Hou JL, Yin YK, et al. Two-year results of a randomized, phase III comparative trial of telbivudine versus lamivudine in Chinese patients. Hepatol Int. 2014;8(1):72–82.
Hou J, Yin YK, Xu D, et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: results at 1 year of a randomized, double-blind trial. Hepatology. 2008;47(2):447–54.
Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136(2):486–95.
Wang Y, Thongsawat S, Gane EJ, et al. Efficacy and safety of continuous 4-year telbivudine treatment in patients with chronic hepatitis B. J Viral Hepat. 2013;20(4):e37–46.
Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–55.
Hou JL, Gao ZL, Xie Q, et al. Tenofovir disoproxil fumarate vs adefovir dipivoxil in Chinese patients with chronic hepatitis B after 48 weeks: a randomized controlled trial. J Viral Hepat. 2015;22(2):85–93.
Minde Z, Yimin M, Guangbi Y, et al. Five years of treatment with adefovir dipivoxil in Chinese patients with HBeAg-positive chronic hepatitis B. Liver Int. 2012;32(1):137–46.
Marcellin P, Chang TT, Lim SG, et al. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48(3):750–8.
Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–10.
Yao GBRH, Xu DZ, Zhou XQ, et al. Results of 3 years of continuous entecavir treatment in nucleos(t)ide-naive chronic hepatits B patients. Chin J Hepatol. 2009;17(12):881–6.
Yuen MF, Seto WK, Fung J, et al. Three years of continuous entecavir therapy in treatment-naive chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106(7):1264–71.
Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49(5):1503–14.
Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140(1):132–43.
Buti M, Tsai N, Petersen J, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60(5):1457–64.
Marcellin P, Gane E, Flisiak R, et al. Long term treatment with tenofovir disoproxil fumarate for chronic hepatitis b infection is safe and well tolerated and associated with durable virologic response with no detectable resistance: 8 year results from two phase 3 trials. Hepatology. 2014;60:313A–4A.
Kitrinos KM, Corsa A, Liu Y, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59(2):434–42.
Wang J, Shi JP, Zhu MF, et al. The efficacy and safety comparation between nucleoside (acid) analogue initial combination and monotherapy in the treatment of chronic hepatitis B for 144 weeks. Chin J Exp Clin Virol. 2014;28(2):82–4.
Liu YH, Liu L, Peng D, et al. Long-term efficacy and safety of telbivudine as monotherapy and as combination therapy with adefovir dipivoxil in HBeAg-positive chronic hepatitis B patients. Chin J Hepatol. 2014;22(3):181–4.
Jia H, Ding F, Chen J, et al. LAM add-on ADV combination therapy or ETV monotherapy for CHB patients with suboptimal response to ADV. Ann Hepatol. 2015;14(2):175–80.
Li X, Jie Y, You X, et al. Optimized combination therapies with adefovir dipivoxil (ADV) and lamivudine, telbivudine, or entecavir may be effective for chronic hepatitis B patients with a suboptimal response to ADV monotherapy. Int J Clin Exp Med. 2015;8(11):21062–70.
Jiang YM, Liang JC, Chen XX, Gong L, Bao J. Effective analysis of using entecavir in combination with adefovir dipivoxil for a 48-week of chronic hepatitis B patients with lamivudine-resistance. Int J Dig Dis. 2014;34:401–5.
Seo SY, Kim IH, Sohn JY, et al. Long-term efficacy of entecavir plus adefovir combination therapy versus entecavir monotherapy in adefovir refractory chronic hepatitis B patients with prior lamivudine resistance. Intervirology. 2014;57(1):8–16.
Jeon JW, Shin HP, Lee JI, et al. Efficacy of entecavir and adefovir combination therapy for patients with lamivudine- and entecavir-resistant chronic hepatitis B. Dig Dis Sci. 2012;57(5):1358–65.
Yao G, Chen C, Lu W, et al. Efficacy and safety of entecavir compared to lamivudine in nucleoside-naive patients with chronic hepatitis B: a randomized double-blind trial in China. Hepatol Int. 2007;1(3):365–72.
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131(6):1743–51.
Lok AS, Trinh H, Carosi G, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naive patients with chronic hepatitis B. Gastroenterology. 2012;143(3):619–628e1.
Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354(10):1011–20.
Yu JH, Shi JP, Wu J, et al. Efficacy and safety of lamivudine plus adefovir combination therapy and entecavir monotherapy for chronic hepatitis B patients. Chin J Hepatol. 2011;19(2):88–92.
Dan YY, Wong JB, Hamid SS, et al. Consensus cost-effectiveness model for treatment of chronic hepatitis B in Asia Pacific countries. Hepatol Int. 2014;8(3):382–94.
He J, Bowen JM, Xie F, Goeree R. Cost-effectiveness analysis of antiviral treatments for HBeAg-positive chronic hepatitis B in Canada. Value Health. 2012;15(6):894–906.
Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73.
Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130(3):678–86.
Mommeja-Marin H, Mondou E, Blum MR, Rousseau F. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology. 2003;37(6):1309–19.
Chen L. A cost-effectiveness study on Avarbose to treat type 2 diabetes. Available from: http://d.wanfangdata.com.cn/Thesis/D446438. Accessed Nov 2015.
Pharmacoeconomics Committee of Chinese Pharmaceutical Association. China guidelines for pharmacoeconomic evaluations. Chin J Pham Econ. 2010;5:5–43.
Yao GB, Cui ZY, Yao JL, et al. Chronic hepatitis B treated with domestic manufactured lamivudine in 2200 patients: a phase IV study. Chin J Hepatol. 2003;11(2):103–8.
Wang HY, Guo RC. Comparative study on the domestic and imported entecavir. J Pharm Res. 2014;33:100–2.
Li KJ, Lou XZ, Xia H, et al. A clinical study of home-made adefovir dipivoxil in the 48-week treatment of patients with HBeAg-positive chronic hepatitis B. Modern Prev Med. 2010;37:3386–7.
The price of Chinese medicine. Available from: http://www.zgyyjgw.com/front/cn/retailPrice. Accessed Jun 2016.
National Bureau of Staristics of China. Available from: http://data.stats.gov.cn/easyquery.htm?cn=C01. Accessed Sep 2015.
Hu M, Chen W. Assessment of total economic burden of chronic hepatitis B (CHB)-related diseases in Beijing and Guangzhou, China. Value Health. 2009;12(Suppl. 3):S89–92.
Gao Q. Economic analysis for liver and renal transplant in a transplant center. Available from: http://d.wanfangdata.com.cn/Thesis/Y1326546. Accessed Jun 2016.
Levy AR, Kowdley KV, Iloeje U, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health. 2008;11(3):527–38.
Bermingham SL, Hughes R, Fenu E, et al. Cost-effectiveness analysis of alternative antiviral strategies for the treatment of HBeAg-positive and HBeAg-negative chronic hepatitis B in the United Kingdom. Value Health. 2015;18(6):800–9.
Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–108.
Chen JG, Zhang SW. Liver cancer epidemic in China: past, present and future. Semin Cancer Biol. 2011;21(1):59–69.
Zhang S, Ma Q, Liang S, et al. Annual economic burden of hepatitis B virus-related diseases among hospitalized patients in twelve cities in China. J Viral Hepat. 2016;23(3):202–10.
Yao GB, Zhu M, Cui ZY, et al. A 7-year study of lamivudine therapy for hepatitis B virus e antigen-positive chronic hepatitis B patients in China. J Dig Dis. 2009;10(2):131–7.
Papatheodoridis GV, Dimou E, Laras A, et al. Course of virologic breakthroughs under long-term lamivudine in HBeAg-negative precore mutant HBV liver disease. Hepatology. 2002;36(1):219–26.
Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology. 2004;40(4):883–91.
Rizzetto M, Tassopoulos NC, Goldin RD, et al. Extended lamivudine treatment in patients with HBeAg-negative chronic hepatitis B. J Hepatol. 2005;42(2):173–9.
Gish R, Jia JD, Locarnini S, Zoulim F. Selection of chronic hepatitis B therapy with high barrier to resistance. Lancet Infect Dis. 2012;12(4):341–53.
Dakin H, Bentley A, Dusheiko G. Cost-utility analysis of tenofovir disoproxil fumarate in the treatment of chronic hepatitis B. Value Health. 2010;13(8):922–33.
Buti M, Brosa M, Casado MA, et al. Modeling the cost-effectiveness of different oral antiviral therapies in patients with chronic hepatitis B. J Hepatol. 2009;51(4):640–6.
Qiu Q, Duan XW, Li Y, et al. Impact of partial reimbursement on hepatitis B antiviral utilization and adherence. World J Gastroenterol. 2015;21(32):9588–97.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The National Natural Science Foundation of China (Grant No. 71573059) provided financial support for this work. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Conflict of interest
All authors have no conflicts of interest to declare.
Additional information
K. Lai and C. Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lai, K., Zhang, C., Ke, W. et al. Cost-Effectiveness Comparison Between the Response-Guided Therapies and Monotherapies of Nucleos(t)ide Analogues for Chronic Hepatitis B Patients in China. Clin Drug Investig 37, 233–247 (2017). https://doi.org/10.1007/s40261-016-0486-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0486-8