Abstract
Background/Aims
Chronic hepatitis B has a high prevalence (>8%) in China. We compared the safety and efficacy of entecavir with that of lamivudine for the treatment of patients with chronic hepatitis B in China.
Methods
A total of 519 nucleoside-naive Chinese patients with chronic hepatitis B were randomized (1:1) and treated with entecavir 0.5 mg/d or lamivudine 100 mg/d. The primary endpoint was serum HBV DNA <0.7 MEq/ml by bDNA assay and alanine aminotransferase <1.25 × upper limit of normal (ULN) at week 48. Patients with missing week 48 measurements were considered non-responders.
Results
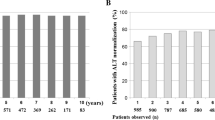
About 90% (231/258) of entecavir-treated versus 67% (174/261) of lamivudine-treated patients achieved the primary endpoint (P < 0.0001). The mean reduction from baseline in HBV DNA was greater with entecavir than lamivudine (5.90 vs. 4.33 log10 copies/ml, P < 0.0001). Greater proportions of entecavir-treated patients achieved undetectable HBV DNA (<300 copies/ml) by polymerase chain reaction assay (76% vs. 43%, P < 0.0001) and alanine aminotransferase normalization (≤1 × ULN, 90% vs. 78%, P = 0.0003). Entecavir and lamivudine achieved comparable rates of HBeAg seroconversion (15% and 18%, respectively). Safety was comparable between the two treatments.
Conclusions
For nucleoside-naïve Chinese patients with chronic hepatitis B, entecavir achieves superior virological and biochemical benefit over lamivudine, with a comparable safety profile.


Similar content being viewed by others
Abbreviations
- ALT:
-
alanine aminotransferase
- bDNA:
-
branched-chain DNA
- HBeAg:
-
hepatitis B e antigen
- HBV:
-
hepatitis B virus
- PCR:
-
polymerase chain reaction
- ULN:
-
upper limit of normal
References
Hepatitis B Foundation [Web site]. Available at: http://www.hepb.org/02-0360.hepb. Accessed January 19, 2005.
Sun Z, Ming L, Zhu X, Lu J. Prevention and control of hepatitis B in China. J Med Virol 2002;67:447–50.
Lee WM. Hepatitis B virus infection. N Engl J Med 1997;337:1733–45.
Liaw YF, Sung JJY, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521–31.
Lai CL, Dienstag J, Schiff E, Leung NWY, Atkins M, Hunt C, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis 2003;36:687–96.
Colonno RJ, Genovesi EV, Medina I, Lamb L, Durham SK, Huang ML, et al. Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J Infect Dis 2001;184:1236–45.
Innaimo SF, Seifer M, Bisacchi GS, Standring DN, Zahler R, Colonno RJ. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother 1997;41:1444–8.
Lai C-L, Rosmawati M, Lao J, Van Vlierberghe H, Anderson FH, Thomas N, et al. Entecavir is superior to lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infection. Gastroenterology 2002;123:1831–8.
Yao GB, Xu DZ, Wang BE, Zhou X-Q, Lei B-J, Zhang DF, et al. A phase II study in China of the safety and antiviral activity of entecavir in adults with chronic hepatitis B infection. Hepatology 2003;38(suppl 1):711A.
Chang T-T, Gish RG, de Man R, Gadano A, Sollano J, Chao Y-C, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001–10.
Lai C-L, Shouval D, Lok AS, Chang T-T, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011–20.
Lok ASF, McMohan BJ. Chronic hepatitis B. Hepatology 2007;45:507–39.
Iloeje UH, Yang H-I, Su J, Jen C-L, You S-L, Chen C-J. Predicting cirrhosis risk based upon the level of circulating hepatitis B viral load. Gastroenterology 2006;130:678–86.
Chen C-J, Yang H-I, Su J, Jen C-L, You S-L, Lu S-N, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65–73.
Liaw YF, Leung N, Guan R, Lau GKK, Merican I, McCaughan G, et al. Asian-Pacific consensus statement on the management of chronic hepatiitis B: a 2005 update. Liver Int 2005;25:472–89.
Keeffe EB, Dieterich DT, Han S-HB, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol 2006;8:936–62.
Marcellin P, Chang T-T, Lim SG, Tong MJ, Sievert W, Shiffman ML, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003;348:808–16.
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang T-T, Kitis G, Rizzetto M, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003;348:800–7.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Other members of the study group
In addition to the authors, the AI463023 Study Group included the following investigators: Shuqing Cai, Weixiong Chen, Yagang Chen, Jinlin Hou, Darong Hu, Yanyan Ji, Jidong Jia, Lin Lin, Aimin Sun, Deying Tian, Mobin Wan, Qinhuan Wang, Lai Wei, Weimin Xu, Youkuan Yin, Minde Zeng, Lingxia Zhang, Shuncai Zhang, Xiaqiu Zhou, Limin Zhu.
An erratum to this article is available at http://dx.doi.org/10.1007/s12072-007-9043-0.
Rights and permissions
About this article
Cite this article
Yao, G., Chen, C., Lu, W. et al. Efficacy and safety of entecavir compared to lamivudine in nucleoside-naïve patients with chronic hepatitis B: a randomized double-blind trial in China. Hepatol Int 1, 365–372 (2007). https://doi.org/10.1007/s12072-007-9009-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-007-9009-2