Abstract
Background and Objective
The cost-effectiveness of highly effective, but costly, peg-interferon (peg-IFN) treatment for chronic hepatitis B (CHB) infections in China is unknown. Endemic hepatitis D virus (HDV) may also modify the effectiveness of any HBV treatment option. The objective of this study is to determine the best antiviral treatment from a societal perspective in the Chinese population, which contains a mix of HBV and HDV infections.
Methods
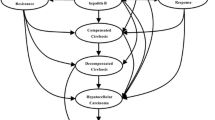
A Markov model is developed to simulate the clinical course of CHB and chronic hepatitis D (CHD) individuals. For a hypothetical Chinese cohort of 10,000 individuals aged 30–60 years, cost-utility analysis is performed for therapies with: lamivudine, adefovir, telbivudine, entecavir, IFN and Peg-IFN. Costs and quality-adjusted life-years (QALYs) are discounted at 3 % annually. A one-way sensitivity analysis is also conducted.
Results
Lamivudine, adefovir, telbivudine, and entecavir are all cost-effective treatments compared to palliative care at an incremental cost-effectiveness ratio (ICER) of −$418, −$197, −$443 and −$317 per QALY, respectively (2015 US dollars). Peg-IFN yields a maximum 156,000 QALYs with an ICER of $1149 per QALY while IFN results in the highest cumulative mortality of 48 % along with the lowest QALY gained. Probabilistic sensitivity analyses confirm that only Peg-IFN and ETV are the only two cost-effective options at the current willingness-to-pay (WTP) of $12,000 in China. However, entecavir has a higher probability of being cost-effective than Peg-IFN at current WTP for all age groups.
Conclusions
Peg-IFN generates maximum QALYs compared to lamivudine, adefovir, telbivudine and interferon, and presents itself as a cost-effective option at current WTP. Alternatively entecavir can be used in China, generating 10 % lower QALYs than Peg-IFN but costing less than palliative care.





Similar content being viewed by others
References
Toy M, Salomon JA, Hao J, Honglian G, Wang H, Wang J, et al. Population health impact and cost-effectiveness of monitoring inactive chronic hepatitis B and treating eligible patients in Shanghai, China. Hepatology (Baltimore, Md). 2013;60(1):46–55.
Qiu Q, Li Y, X-w Duan, L-k Yang, Chen Y, Li H, et al. Impact of a new reimbursement program on hepatitis B antiviral medication cost and utilization in Beijing, China. PLoS One. 2014;9(10):e109652.
Hutton DW, So SK, Brandeau ML. Cost-effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology (Baltimore, Md). 2010;51(2):405–14.
Liao B, Zhang F, Lin S, He H, Liu Y, Zhang J, et al. Epidemiological, clinical and histological characteristics of HBV/HDV co-infection: a retrospective cross-sectional study in Guangdong, China. PLoS One. 2014;9(12):e115888.
Abbas Z, Jafri W, Raza S. Hepatitis D: scenario in the Asia-Pacific region. World J Gastroenterol WJG. 2010;16(5):554–62.
Lin HH, Shin-Jung Lee S, Yu ML, Chang TT, Su CW, Hu BS, et al. Changing hepatitis D virus epidemiology in a hepatitis B virus endemic area with a national vaccination program. Hepatology (Baltimore, Md). 2015;61(6):1870–9.
Wranke A, Heidrich B, Ernst S, Calle Serrano B, Caruntu FA, Curescu MG, et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One. 2014;9(7):e101002.
Wu B, Li T, Chen H, Shen J. Cost-effectiveness of nucleoside analog therapy for hepatitis B in China: a Markov analysis. Value Health. 2010;13(5):592–600.
Zheng X, Wang J, Yang D. Antiviral therapy for chronic hepatitis B in China. Med Microbiol Immunol. 2015;204(1):115–20.
Wu B, Shen J, Cheng H. Cost-effectiveness analysis of different rescue therapies in patients with lamivudine-resistant chronic hepatitis B in China. BMC Health Serv Res. 2012;12:385.
Wang J. Clinical utility of entecavir for chronic hepatitis B in Chinese patients. Drug Design Dev Ther. 2014;8:13–24.
Lui YY, Tsoi KK, Wong VW, Kao JH, Hou JL, Teo EK, Mohamed R, Piratvisuth T, Han KH, Mihm U, Wong GLH, Chan HLY. Cost-effectiveness analysis of roadmap models in chronic hepatitis B using tenofovir as the rescue therapy. Antivir Ther. 2010;15:145–55.
Lu J, Xu A, Wang J, Zhang L, Song L, Li R, et al. Direct economic burden of hepatitis B virus related diseases: evidence from Shandong, China. BMC Health Serv Res. 2013;13(1):37.
Zhang C, Ke W, Gao Y, Zhou S, Liu L, Ye X, et al. Cost-effectiveness analysis of antiviral therapies for hepatitis B e antigen-positive chronic hepatitis B patients in China. Clin Drug Investig. 2015;35(3):197–209.
Tantai N, Chaikledkaew U, Tanwandee T, Werayingyong P, Teerawattananon Y. A cost-utility analysis of drug treatments in patients with HBeAg-positive chronic hepatitis B in Thailand. BMC Health Serv Res. 2014;14:170.
Tu HAT, Woerdenbag HJ, Riewpaiboon A, Kane S, Le DM, Postma MJ, et al. Cost of illness of chronic hepatitis B infection in Vietnam. Value Health Reg Issues. 2012;1(1):23–8.
Sullivan SD, Veenstra DL, Chen PJ, Chang TT, Chuang WL, Tsai C, et al. Cost-effectiveness of peginterferon alpha-2a compared to lamivudine treatment in patients with hepatitis B e antigen positive chronic hepatitis B in Taiwan. J Gastroenterol Hepatol. 2007;22(9):1494–9.
Noureddin M, Gish R. Hepatitis delta: epidemiology, diagnosis and management 36 years after discovery. Curr Gastroenterol Rep. 2014;16(1):365.
James G, Sidhu P, Raza M. First report of successful clearance of hepatitis b and d co-infection with tenofovir monotherapy. Hepatology (Baltimore, Md). 2014;62(1):317–8.
Rizzetto M, Smedile A. Peg-interferon therapy of chronic hepatitis D; in need of revision. Hepatology (Baltimore, Md). 2014.
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatitis. 2004;11(2):97–107.
Abbas Z, Khan MA, Salih M, Jafri W. Interferon alpha for chronic hepatitis D. Cochrane Database Syst Rev. 2011;12:CD006002.
Keshvari M, Alavian SM, Sharafi H, Karimi G, Gholami Fesharaki M. Interferon alpha-2b therapy in chronic hepatitis delta. Hepatitis Mon. 2014;14(3):e15729.
Tugui L, Dumitru M, Iacob S, Gheorghe L, Preda C, Dinu I, et al. The efficacy of the Peginterferon treatment in chronic hepatitis HDV and compensate liver cirrhosis. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi. 2014;118(2):368–75.
Gozlan J, Lacombe K, Gault E, Raguin G, Girard PM. Complete cure of HBV-HDV co-infection after 24 weeks of combination therapy with pegylated interferon and ribavirin in a patient co-infected with HBV/HCV/HDV/HIV. J Hepatol. 2009;50(2):432–4.
Erhardt A, Gerlich W, Starke C, Wend U, Donner A, Sagir A, et al. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int Off J Int Assoc Study Liver. 2006;26(7):805–10.
Lau DT, Kleiner DE, Park Y, Di Bisceglie AM, Hoofnagle JH. Resolution of chronic delta hepatitis after 12 years of interferon alfa therapy. Gastroenterology. 1999;117(5):1229–33.
Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepatitis. 2003;10(4):298–305.
Wiens A, Lenzi L, Venson R, Pedroso MLA, Correr CJ, Pontarolo R. Economic evaluation of treatments for chronic hepatitis B. Braz J Infect Dis. 2013;17:418–26.
Castelnau C, Le Gal F, Ripault MP, Gordien E, Martinot-Peignoux M, Boyer N, et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology (Baltimore, Md). 2006;44(3):728–35.
Wedemeyer H, Hardtke S, Manns MP. Treatment of hepatitis delta. Clin Liver Dis. 2013;2(6):237–9.
Heidrich B, Yurdaydin C, Kabacam G, Ratsch BA, Zachou K, Bremer B, et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology (Baltimore, Md). 2014;60(1):87–97.
Gheorghe L, Iacob S, Simionov I, Vadan R, Gheorghe C, Iacob R, et al. Natural history of compensated viral B and D cirrhosis. Rom J Gastroenterol. 2005;14(4):329–35.
Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. Gut. 2000;46(3):420–6.
Heidrich B, Serrano BC, Idilman R, Kabacam G, Bremer B, Raupach R, et al. HBeAg-positive hepatitis delta: virological patterns and clinical long-term outcome. Liver Int Off J Int Assoc Study Liver. 2012;32(9):1415–25.
Colombo GL, Gaeta GB, Vigano M, Di Matteo S. A cost-effectiveness analysis of different therapies in patients with chronic hepatitis B in Italy. ClinicoEconomics Outcomes Res CEOR. 2011;3:37–46.
Lee S, Kwon CH, Moon HH, Kim TS, Roh Y, Song S, et al. Antiviral treatment for hepatitis B virus recurrence following liver transplantation. Clin Transpl. 2013;27(5):E597–604.
Marsman WA, Wiesner RH, Batts KP, Poterucha JJ, Porayko MK, Niesters HG, et al. Fulminant hepatitis B virus: recurrence after liver transplantation in two patients also infected with hepatitis delta virus. Hepatology (Baltimore, Md). 1997;25(2):434–8.
Ottobrelli A, Marzano A, Smedile A, Recchia S, Salizzoni M, Cornu C, et al. Patterns of hepatitis delta virus reinfection and disease in liver transplantation. Gastroenterology. 1991;101(6):1649–55.
Kanwal F, Farid M, Martin P, Chen G, Gralnek IM, Dulai GS, et al. Treatment alternatives for hepatitis B cirrhosis: a cost-effectiveness analysis. Am J Gastroenterol. 2006;101(9):2076–89.
Romeo R. Hepatitis delta: natural history and outcome. Clin Liver Dis. 2013;2(6):235–6.
Rizzetto M, Chiaberge E, Negro F, Di Giacomo C, Cortesini R, Doglia M, et al. Liver transplantation in hepatitis delta virus disease. Lancet. 1987;330(8557):469–71.
Tang C-M, Yau TO, Yu J. Management of chronic hepatitis B infection: current treatment guidelines, challenges, and new developments. World J Gastroenterol WJG. 2014;20(20):6262–78.
Niro GA, Rosina F, Rizzetto M. Treatment of hepatitis D. J Viral Hepatitis. 2005;12(1):2–9.
Ciancio A, Rizzetto M. Chronic hepatitis D at a standstill: where do we go from here? Nat Rev Gastroenterol Hepatol. 2013;11(1):68–71.
Heller T, Rotman Y, Koh C, Clark S, Haynes-Williams V, Chang R, et al. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther. 2014;40(1):93–104.
Shen L, Gu Y, Sun L, Yang Y, Wang F, Li Y, et al. Development of a hepatitis delta virus antibody assay for study of the prevalence of HDV among individuals infected with hepatitis B virus in China. J Med Virol. 2012;84(3):445–9.
Goyal A, Murray JM. The impact of vaccination and antiviral therapy on hepatitis B and hepatitis D epidemiology. PLoS One. 2014;9(10):e110143.
Fernandez-Montero JV, Vispo E, Barreiro P, Sierra-Enguita R, de Mendoza C, Labarga P, et al. Hepatitis delta is a major determinant of liver decompensation events and death in HIV-infected patients. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58(11):1549–53.
Goyal A, Murray JM. Recognizing the impact of endemic hepatitis D virus on hepatitis B virus eradication. Unpublished work; 2015.
Hutton DW, Brandeau ML. Too much of a good thing? When to stop catch-up vaccination. Med Decis Making Int J Soc Med Decis Making. 2013;33(7):920–36.
Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med. 2007;147(7):460–9.
Hutton DW, Brandeau ML, So SK. Doing good with good OR: supporting cost-effective hepatitis B interventions. Interfaces. 2011;41(3):289–300.
Wei L, Hu S, Hou J, Liu G, Ren H, Duan Z, et al. A novel estimation of the impact of treatment with entecavir on long-term mortality, morbidity, and health care costs of chronic hepatitis B in China. Value Health Reg Issues. 2013;2(1):48–56.
Ke W, Liu L, Zhang C, Ye X, Gao Y, Zhou S, et al. Comparison of efficacy and safety of tenofovir and entecavir in chronic hepatitis B virus infection: a systematic review and meta-analysis. PLoS One. 2014;9(6):e98865.
Toy M, Onder FO, Idilman R, Kabacam G, Richardus JH, Bozdayi M, et al. The cost-effectiveness of treating chronic hepatitis B patients in a median endemic and middle income country. Eur J Health Econ. 2012;13(5):663–76.
Jablkowski MS, Diculescu M, Janssen HL, et al. The safety and efficacy of entecavir and tenofovir combination therapy for chronic hepatitis B in patients with previous nucleos(t)ide treatment failure. In: 64th annual meeting of the American Association for the Study of Liver Diseases; 2013, Washington, DC; 2013.
Wu C, Dunn W. Is it worthy of switching to PegIFN alfa-2a in patients achieving virological suppression with entecavir? J Hepatol. 2015;62(6):1439–40.
Alvarado-Mora MV, Locarnini S, Rizzetto M, Pinho JR. An update on HDV: virology, pathogenesis and treatment. Antivir Ther. 2013;18(3 Pt B):541–8.
Ding S, Robek MD. Cytidine deamination and cccDNA degradation: a new approach for curing HBV? Hepatology (Baltimore, Md). 2014;60(6):2118–21.
Koh C, Yurdaydin C, Cooper S, Cory D, Dahari H, Haynes-Williams V, et al. Prenylation inhibition with lonafarnib decreases hepatitis D levels in humans. Hepatology (Baltimore, Md). 2014;60:92A–196A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study.
Conflict of interest
Ashish Goyal and John M. Murray have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Goyal, A., Murray, J.M. Cost-Effectiveness of Peg-Interferon, Interferon and Oral Nucleoside Analogues in the Treatment of Chronic Hepatitis B and D Infections in China. Clin Drug Investig 36, 637–648 (2016). https://doi.org/10.1007/s40261-016-0409-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0409-8