Abstract
Background and Objective
Vortioxetine and duloxetine are two new antidepressant drugs that have been used clinically in the treatment of major depressive disorder (MDD). The objectives of this meta-analysis were to evaluate the efficacy and tolerability of vortioxetine compared with duloxetine in MDD.
Methods
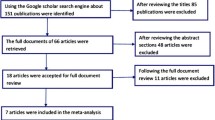
Randomized controlled trials (RCTs) published in PubMed, EMBASE, Web of Science, and ClinicalTrials.gov were systematically reviewed to compare vortioxetine with duloxetine in terms of efficacy and tolerability in patients with MDD. Results were expressed as the risk ratio (RR) with 95 % confidence intervals (CIs), and weighted mean difference (WMD). Pooled estimates were calculated by using a fixed-effects model or a randomized-effects model, depending on the heterogeneity among studies.
Results
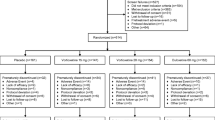
A total of five RCTs involving 2287 patients met the inclusion criteria and were included in this meta-analysis. Pooled results showed that duloxetine was associated with a higher response rate than vortioxetine, as well as showing a similar remission rate with vortioxetine. The changes from baseline in the Montgomery–Åsberg Depression Rating Scale (MADRS), 24-item Hamilton Rating Scale for Depression (HAM–D24), Clinical Global Impression–Improvement scale (CGI–I), CGI–Severity scale (CGI–S), Sheehan Disability Scale (SDS), and Hamilton Anxiety Rating Scale (HAM–A) scores were significantly greater in the duloxetine group than in the vortioxetine group. The incidence of treatment-emergent adverse events was significantly higher in the duloxetine group than in the vortioxetine group.
Conclusion
Duloxetine was more effective but less well-tolerated than vortioxetine in MDD. Considering the potential limitations of this meta-analysis, more large-scale RCTs are needed to confirm these findings.




Similar content being viewed by others
References
Greden JF. The burden of recurrent depression: causes, consequences, and future prospects. J Clin Psychiatry. 2001;62(Suppl 22):5–9.
Rouillon F, Gorwood P. The use of lithium to augment antidepressant medication. J Clin Psychiatry. 1998;59(Suppl 5):32–9 (discussion 40–41).
Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin N Am. 1996;19(2):179–200.
Tranter R, O’Donovan C, Chandarana P, Kennedy S. Prevalence and outcome of partial remission in depression. J Psychiatry Neurosci. 2002;27(4):241–7.
Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2–3):97–108.
Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171–80.
Papakostas GI. The efficacy, tolerability, and safety of contemporary antidepressants. J Clin Psychiatry. 2010;71(Suppl E1)):e03.
Masand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289–304.
Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE, et al. Innovative approaches for the development of antidepressant drugs: current and future strategies. NeuroRx. 2005;2(4):590–611.
Henigsberg N, Mahableshwarkar AR, Jacobsen P, Chen Y, Thase ME. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73(7):953–9.
Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871–80.
Goldstein DJ, Mallinckrodt C, Lu Y, Demitrack MA. Duloxetine in the treatment of major depressive disorder: a double-blind clinical trial. J Clin Psychiatry. 2002;63(3):225–31.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135(11):982–9.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138–49.
Baldwin DS, Loft H, Dragheim M. A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD). Eur Neuropsychopharmacol. 2012;22(7):482–91.
Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215–23.
Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. 2013;29(3):217–26.
Mahableshwarkar AR, Jacobsen PL, Chen Y, Serenko M, Trivedi MH. A randomized, double-blind, duloxetine-referenced study comparing efficacy and tolerability of 2 fixed doses of vortioxetine in the acute treatment of adults with MDD. Psychopharmacology. 2015;232(12):2061–70.
Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(5):589–600.
Mahableshwarkar AR, Jacobsen PL, Serenko M, Chen Y, Trivedi MH. A randomized, double-blind, placebo-controlled study of the efficacy and safety of 2 doses of vortioxetine in adults with major depressive disorder. J Clin Psychiatry. 2015;76(5):583–91.
Khin NA, Chen YF, Yang Y, Yang P, Laughren TP. Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications. J Clin Psychiatry. 2011;72(4):464–72.
Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–7.
Khan A, Detke M, Khan SR, Mallinckrodt C. Placebo response and antidepressant clinical trial outcome. J Nerv Ment Dis. 2003;191(4):211–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in this study.
Conflict of interest
Guangjian Li, Xu Wang, Dihui Ma declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, G., Wang, X. & Ma, D. Vortioxetine versus Duloxetine in the Treatment of Patients with Major Depressive Disorder: A Meta-Analysis of Randomized Controlled Trials. Clin Drug Investig 36, 509–517 (2016). https://doi.org/10.1007/s40261-016-0396-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0396-9