Abstract
Background and Objective
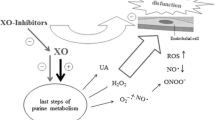
There is growing evidence of an association between high uric acid (UA) levels and cardiovascular disease (CVD). We hypothesized that febuxostat, a xanthine oxidase inhibitor, may be associated with suppressing the renin-angiotensin-aldosterone system (RAAS) and improving renal function in hyperurecemic patients with hypertension.
Methods
We conducted a 6-month prospective study in which we randomized hypertensive hyperuricemic patients to either a febuxostat group (n = 30) or a control group (n = 30). The dose of febuxostat was adjusted to maintain the serum UA level at <6.0 mg/dL.
Results
In the febuxostat group, the plasma renin activity (PRA), plasma aldosterone concentration (PAC), and serum UA level significantly decreased by 33 % (p = 0.0012), 14 % (p = 0.001), and 29 % (p < 0.0001), respectively. The estimated glomerular filtration rate (eGFR) significantly increased by 5.5 % (p = 0.001). Similar changes were not observed in the control group. Furthermore, a significant correlation was observed between the percent changes in the serum UA levels and the percent changes in the PRA (r = 0.277, p = 0.033), PAC (r = 0.310, p = 0.016), serum blood urea nitrogen levels (r = 0.434, p = 0.0005), serum creatinine levels (r = 0.413, p = 0.002), and eGFR (r = −0.474, p = 0.0001).
Conclusions
These results support the hypothesis that febuxostat might not only reduce serum UA levels but also suppress RAAS and improve renal function in hyperuricemic patients with hypertension, possibly leading to prevention of CVD.




Similar content being viewed by others
References
Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–93.
Franse LV, Pahor M, Di Bari M, et al. Serum uric acid, diuretic treatment and risk of cardiovascular events in the Systolic Hypertension in the Elderly Program (SHEP). J Hypertens. 2000;18:1149–54.
Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension. 2000;36:1072–8.
Krishnan E. Hyperuricemia and incident heart failure. Circ Heart Fail. 2009;2:556–62.
Noman A, Ang DS, Ogston S, Lang CC, Struthers AD. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375:2161–7.
Collaboration Blood Pressure Lowering Treatment Trialists’, Turnbull F, Neal B, Pfeffer M, et al. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25:951–8.
Ripley E. Complementary effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in slowing the progression of chronic kidney disease. Am Heart J. 2009;157:S7–16.
Malik UZ, Hundley NJ, Romero G, Radi R, Freeman BA, Tarpey MM, et al. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic Biol Med. 2011;51:179–84.
Ye P, Yang S, Zhang W, et al. Efficacy and tolerability of febuxostat in hyperuricemic patients with or without gout: a systematic review and meta-analysis. Clin Ther. 2013;35:180–9.
Hosoya T, Kimura K, Itoh S, et al. The effect of febuxostat to prevent a further reduction in renal function of patients with hyperuricemia who have never had gout and are complicated by chronic kidney disease stage 3: study protocol for a multicenter randomized controlled study. Trials. 2014;15:26.
Saito I, Saruta T, Kondo K, et al. Serum uric acid and the renin-angiotensin system in hypertension. J Am Geriatr Soc. 1978;26:241–7.
Gruskin AB. The adolescent with essential hypertension. Am J Kidney Dis. 1985;6:86–90.
Yu MA, Sánchez-Lozada LG, Johnson RJ, et al. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28:1234–42.
Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension:a randomized trial. JAMA. 2008;300:924–32.
Talaat KM, El-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27:435–40.
Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol. 2011;17:7–13.
Sanchez-Lozada LG, Tapia E, Santamaria J, Avila-Casado C, Soto V, Nepomuceno T, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–47.
Sanchez-Lozada LG, Tapia E, Avila-Casado C, Soto V, Franco M, Santamaria J, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol. 2002;283:F1105–10.
Ogihara T, Kikuchi K, Matsuoka H, Japanese Society of Hypertension Committee, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2009). Hypertens Res. 2009;32:3–107.
Høieggen A, Alderman MH, Kjeldsen SE, LIFE Study Group, et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–9.
Matsuo S, Imai E, Horio M, Collaborators developing the Japanese equation for estimated GFR, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–6.
Herlitz LC, D’Agati VD, Markowitz GS. Crystalline nephropathies. Arch Pathol Lab Med. 2012;136:713–20.
Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med. 2004;140:167–74.
Soltani Z, Rasheed K, Kapusta DR, et al. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013;15:175–81.
Sánchez-Lozada LG, Tapia E, Jiménez A, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:F423–9.
Maejima I, Takahashi A, Omori H, et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 2013;32:2336–47.
Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21.
Aroor AR, McKarns S, Demarco VG, et al. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62:1543–52.
Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–7.
Zhang J, Zhang Y, Deng W, et al. Elevated serum uric acid is associated with angiotensinogen in obese patients with untreated hypertension. J Clin Hypertens (Greenwich). 2014;16:569–74.
Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–9.
Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–33.
Alderman MH, Cohen H, Madhavan S, et al. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–50.
Cohn JN, Anand IS, Latini R, et al. Valsartan Heart Failure Trial Investigators. Sustained reduction of aldosterone in response to the angiotensin receptor blocker valsartan in patients with chronic heart failure: results from the Valsartan Heart Failure Trial. Circulation. 2003;108:1306–9.
Acknowledgments
We thank all patients and investigators involved in these studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
All patients gave their written informed consent prior to the start of each study.
Rights and permissions
About this article
Cite this article
Tani, S., Nagao, K. & Hirayama, A. Effect of Febuxostat, a Xanthine Oxidase Inhibitor, on Cardiovascular Risk in Hyperuricemic Patients with Hypertension: A Prospective, Open-label, Pilot Study. Clin Drug Investig 35, 823–831 (2015). https://doi.org/10.1007/s40261-015-0349-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0349-8