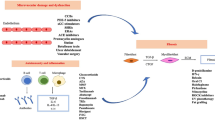
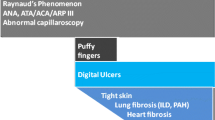
Abstract
Systemic sclerosis (SSc) and morphea are autoimmune sclerosing diseases that cause significant morbidity, and in the case of SSc, mortality. The pathogenesis of both SSc and morphea share vascular dysfunction, auto-reactive T cells and Th2-associated cytokines, such as interleukin 4, and overproduction of transforming growth factor beta (TGFβ). TGFβ stimulates fibroblast collagen and extra-cellular matrix production. Although morphea and SSc have similar pathogenic pathways and histological findings, they are distinct diseases. Recent advances in treatment of morphea, skin sclerosis in SSc, and interstitial lung disease in SSc are focused on targeting known pathogenic pathways.


Similar content being viewed by others
References
Pope JE, et al. State-of-the-art evidence in the treatment of SSc. Nat Rev Rheumatol. 2023;19(4):212–26.
Low AHL, et al. Evaluation of Scleroderma Clinical Trials Consortium training recommendations on modified Rodnan skin score assessment in scleroderma. Int J Rheum Dis. 2019;22(6):1036–40.
Herrick AL, Assassi S, Denton CP. Skin involvement in early diffuse cutaneous SSc: an unmet clinical need. Nat Rev Rheumatol. 2022;18(5):276–85.
Khanna D, et al. The American College of Rheumatology Provisional Composite Response Index for Clinical Trials in Early Diffuse Cutaneous SSc. Arthritis Rheumatol. 2016;68(2):299–311.
Khanna D, et al. New composite endpoint in early diffuse cutaneous SSc: revisiting the provisional American College of Rheumatology Composite Response Index in SSc. Ann Rheum Dis. 2021;80(5):641–50.
Mulcaire-Jones E, et al. Advances in biological and targeted therapies for SSc. Expert Opin Biol Ther. 2023;23(4):325–39.
Di Battista M, et al. SSc: one year in review 2023. Clin Exp Rheumatol. 2023;41(8):1567–74.
Pellar RE, Pope JE. Evidence-based management of SSc: navigating recommendations and guidelines. Semin Arthritis Rheum. 2017;46(6):767–74.
Denton CP, et al. BSR and BHPR guideline for the treatment of SSc. Rheumatology (Oxford). 2016;55(10):1906–10.
Kowal-Bielecka O, et al. Update of EULAR recommendations for the treatment of SSc. Ann Rheum Dis. 2017;76(8):1327–39.
Fernandez-Codina A, et al. Treatment algorithms for SSc according to experts. Arthritis Rheumatol. 2018;70(11):1820–8.
van den Hoogen FH, et al. Comparison of methotrexate with placebo in the treatment of SSc: a 24 week randomized double-blind trial, followed by a 24 week observational trial. Br J Rheumatol. 1996;35(4):364–72.
Pope JE, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 2001;44(6):1351–8.
Namas R, et al. Efficacy of mycophenolate mofetil and oral cyclophosphamide on skin thickness: post hoc analyses from two randomized placebo-controlled trials. Arthritis Care Res (Hoboken). 2018;70(3):439–44.
Tashkin DP, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–19.
Tashkin DP, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66.
Yoshifuji H, et al. Role of rituximab in the treatment of SSc: a literature review. Mod Rheumatol. 2023;33(6):1068–77.
Sullivan KM, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med. 2018;378(1):35–47.
van Bijnen S, et al. Predictive factors for treatment-related mortality and major adverse events after autologous haematopoietic stem cell transplantation for SSc: results of a long-term follow-up multicentre study. Ann Rheum Dis. 2020;79(8):1084–9.
van Laar JM, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous SSc: a randomized clinical trial. JAMA. 2014;311(24):2490–8.
Herrick AL, et al. Treatment outcome in early diffuse cutaneous SSc: the European Scleroderma Observational Study (ESOS). Ann Rheum Dis. 2017;76(7):1207–18.
Bruera S, et al. Stem cell transplantation for SSc. Cochrane Database Syst Rev. 2022;7(7):CD011819.
Burt RK, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for SSc (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378(9790):498–506.
Wollin L, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1434–45.
Distler O, et al. Nintedanib for SSc-associated interstitial lung disease. N Engl J Med. 2019;380(26):2518–28.
Khanna D, et al. Safety and efficacy of subcutaneous tocilizumab in adults with SSc (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387(10038):2630–40.
Khanna D, et al. Tocilizumab in SSc: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020;8(10):963–74.
Khanna D, et al. Abatacept in early diffuse cutaneous SSc: results of a phase II investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 2020;72(1):125–36.
Domsic RT, et al. A data-driven approach finds RNA polymerase III antibody and tendon friction rubs as enrichment tools for early diffuse scleroderma trials. Rheumatology (Oxford). 2023;62(4):1543–51.
Moriana C, et al. JAK inhibitors and SSc: a systematic review of the literature. Autoimmun Rev. 2022;21(10):103168.
Khanna D, et al. Riociguat in patients with early diffuse cutaneous SSc (RISE-SSc): randomised, double-blind, placebo-controlled multicentre trial. Ann Rheum Dis. 2020;79(5):618–25.
Giorgi V, et al. Cannabis and autoimmunity: possible mechanisms of action. Immunotargets Ther. 2021;10:261–71.
Spiera R, et al. Efficacy and safety of lenabasum, a cannabinoid type 2 receptor agonist, in a phase 3 randomized trial in diffuse cutaneous SSc. Arthritis Rheumatol. 2023;75(9):1608–18.
Allanore Y, et al. A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous SSc. Ann Rheum Dis. 2020;79(12):1600–7.
Khanna D, et al. A 24-week, phase IIa, randomized, double-blind, placebo-controlled study of ziritaxestat in early diffuse cutaneous SSc. Arthritis Rheumatol. 2023;75(8):1434–44.
Rice LM, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in SSc patients. J Clin Invest. 2015;125(7):2795–807.
Fukasawa T, et al. Interleukin-17 pathway inhibition with brodalumab in early SSc: analysis of a single-arm, open-label, phase 1 trial. J Am Acad Dermatol. 2023;89(2):366–9.
Guo X, et al. Suppression of T cell activation and collagen accumulation by an anti-IFNAR1 mAb, anifrolumab, in adult patients with SSc. J Invest Dermatol. 2015;135(10):2402–9.
Bergmann C, et al. Treatment of a patient with severe SSc (SSc) using CD19-targeted CAR T cells. Ann Rheum Dis. 2023;82(8):1117–20.
Murray KJ, Laxer RM. Scleroderma in children and adolescents. Rheum Dis Clin N Am. 2002;28(3):603–24.
Peterson LS, et al. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960–1993. J Rheumatol. 1997;24(1):73–80.
Fett N, Werth VP. Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64(2):217–28 (quiz 229–230).
Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18(6):606–13.
Zannin ME, et al. Ocular involvement in children with localised scleroderma: a multi-centre study. Br J Ophthalmol. 2007;91(10):1311–4.
Christen-Zaech S, et al. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59(3):385–96.
Zulian F, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford). 2006;45(5):614–20.
Kister I, et al. Neurologic manifestations of localized scleroderma: a case report and literature review. Neurology. 2008;71(19):1538–45.
Lis-Swiety A, et al. Health-related quality of life and its influencing factors in adult patients with localized scleroderma—a cross-sectional study. Health Qual Life Outcomes. 2020;18(1):133.
Das S, Bernstein I, Jacobe H. Correlates of self-reported quality of life in adults and children with morphea. J Am Acad Dermatol. 2014;70(5):904–10.
Ardalan K, Zigler CK, Torok KS. Predictors of longitudinal quality of life in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2017;69(7):1082–7.
Zigler CK, et al. Exploring the impact of paediatric localized scleroderma on health-related quality of life: focus groups with youth and caregivers. Br J Dermatol. 2020;183(4):692–701.
Arkachaisri T, et al. Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study. Rheumatology (Oxford). 2010;49(2):373–81.
Agazzi A, et al. Reliability of LoSCAT score for activity and tissue damage assessment in a large cohort of patients with juvenile localized scleroderma. Pediatr Rheumatol Online J. 2018;16(1):37.
Kelsey CE, Torok KS. The Localized Scleroderma Cutaneous Assessment Tool: responsiveness to change in a pediatric clinical population. J Am Acad Dermatol. 2013;69(2):214–20.
Skrzypek-Salamon A, et al. Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) adapted for use in adult patients: report from an initial validation study. Health Qual Life Outcomes. 2018;16(1):185.
Teske NM, Jacobe HT. using the localized scleroderma cutaneous assessment tool (loscat) to classify morphoea by severity and identify clinically significant change. Br J Dermatol. 2020;182(2):398–404.
Torok KS. Assigning values to the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) score indicating degree of severity and responsiveness: fostering practical use in clinic and therapeutic studies for morphoea/localized scleroderma. Br J Dermatol. 2020;182(2):272–3.
Fett N, Werth VP. Update on morphea: part II. Outcome measures and treatment. J Am Acad Dermatol. 2011;64(2):231–42 (quiz 243–244).
Kroft EB, et al. Efficacy of topical tacrolimus 0.1% in active plaque morphea: randomized, double-blind, emollient-controlled pilot study. Am J Clin Dermatol. 2009;10(3):181–7.
Badea I, et al. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and SSc. Rheumatology (Oxford). 2009;48(3):213–21.
Cunningham BB, et al. Topical calcipotriene for morphea/linear scleroderma. J Am Acad Dermatol. 1998;39(2 Pt 1):211–5.
Kreuter A, et al. Combined treatment with calcipotriol ointment and low-dose ultraviolet al phototherapy in childhood morphea. Pediatr Dermatol. 2001;18(3):241–5.
Petty AJ, et al. Pilot, open-label, single-arm clinical trial evaluating the efficacy of topical crisaborole for steroid refractory morphea. J Am Acad Dermatol. 2023;89(2):390–2.
Dytoc M, et al. Evaluation of the efficacy and safety of topical imiquimod 5% for plaque-type morphea: a multicenter, prospective, vehicle-controlled trial. J Cutan Med Surg. 2015;19(2):132–9.
Pope E, et al. Topical imiquimod 5% cream for pediatric plaque morphea: a prospective, multiple-baseline, open-label pilot study. Dermatology. 2011;223(4):363–9.
Campione E, et al. Localized morphea treated with imiquimod 5% and dermoscopic assessment of effectiveness. J Dermatolog Treat. 2009;20(1):10–3.
Gruss C, et al. Induction of interstitial collagenase (MMP-1) by UVA-1 phototherapy in morphea fibroblasts. Lancet. 1997;350(9087):1295–6.
Gruss CJ, et al. Effects of low dose ultraviolet A-1 phototherapy on morphea. Photodermatol Photoimmunol Photomed. 2001;17(4):149–55.
Morita A, et al. Ultraviolet al (340–400 nm) phototherapy for scleroderma in SSc. J Am Acad Dermatol. 2000;43(4):670–4.
Yin L, et al. The expression of matrix metalloproteinase-1 mRNA induced by ultraviolet al (340–400 nm) is phototherapy relevant to the glutathione (GSH) content in skin fibroblasts of SSc. J Dermatol. 2003;30(3):173–80.
Grabbe J, et al. High-dose ultraviolet al (UVA1), but not UVA/UVB therapy, decreases IgE-binding cells in lesional skin of patients with atopic eczema. J Invest Dermatol. 1996;107(3):419–22.
Kroft EB, et al. Period of remission after treatment with UVA-1 in sclerodermic skin diseases. J Eur Acad Dermatol Venereol. 2008;22(7):839–44.
El-Mofty M, et al. Suggested mechanisms of action of UVA phototherapy in morphea: a molecular study. Photodermatol Photoimmunol Photomed. 2004;20(2):93–100.
Stege H, et al. High-dose UVA1 radiation therapy for localized scleroderma. J Am Acad Dermatol. 1997;36(6 Pt 1):938–44.
Kerscher M, Dirschka T, Volkenandt M. Treatment of localised scleroderma by UVA1 phototherapy. Lancet. 1995;346(8983):1166.
Andres C, et al. Successful ultraviolet al phototherapy in the treatment of localized scleroderma: a retrospective and prospective study. Br J Dermatol. 2010;162(2):445–7.
Jacobe HT, Cayce R, Nguyen J. UVA1 phototherapy is effective in darker skin: a review of 101 patients of Fitzpatrick skin types I-V. Br J Dermatol. 2008;159(3):691–6.
Kerscher M, et al. PUVA bath photochemotherapy for localized scleroderma. Evaluation of 17 consecutive patients. Arch Dermatol. 1996;132(11):1280–2.
Usmani N, et al. Photochemotherapy for localized morphoea: effect on clinical and molecular markers. Clin Exp Dermatol. 2008;33(6):698–704.
El-Mofty M, et al. Different low doses of broad-band UVA in the treatment of morphea and SSc. Photodermatol Photoimmunol Photomed. 2004;20(3):148–56.
El-Mofty M, et al. Low-dose broad-band UVA in morphea using a new method for evaluation. Photodermatol Photoimmunol Photomed. 2000;16(2):43–9.
Kreuter A, et al. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol. 2006;54(3):440–7.
clinicaltrials.gov. The influence of extracorporeal photopheresis on skin sclerosis. ClinicalTrials.gov ID NCT04752397. Sponsor Charite University, Berlin, Germany. Information provided by Ulrike Blume-Peytavi, MD, Charite University, Berlin, Germany (Responsible Party).
Neustadter JH, et al. Extracorporeal photochemotherapy for generalized deep morphea. Arch Dermatol. 2009;145(2):127–30.
Pileri A, et al. Generalized morphea successfully treated with extracorporeal photochemotherapy (ECP). Dermatol Online J. 2014;20(1):21258.
Uziel Y, et al. Methotrexate and corticosteroid therapy for pediatric localized scleroderma. J Pediatr. 2000;136(1):91–5.
Kreuter A, et al. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol. 2005;141(7):847–52.
Seyger MM, et al. Low-dose methotrexate in the treatment of widespread morphea. J Am Acad Dermatol. 1998;39(2 Pt 1):220–5.
Weibel L, et al. Prospective evaluation of treatment response and disease reversibility of paediatric localized scleroderma (morphoea) to steroids and methotrexate using multi-modal imaging. J Eur Acad Dermatol Venereol. 2020;34(7):1609–16.
Fitch PG, et al. Treatment of pediatric localized scleroderma with methotrexate. J Rheumatol. 2006;33(3):609–14.
Weibel L, et al. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155(5):1013–20.
Kroft EB, et al. Effectiveness, side-effects and period of remission after treatment with methotrexate in localized scleroderma and related sclerotic skin diseases: an inception cohort study. Br J Dermatol. 2009;160(5):1075–82.
Martini G, et al. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology (Oxford). 2009;48(11):1410–3.
Martini G, et al. Mycophenolate mofetil for methotrexate-resistant juvenile localized scleroderma. Rheumatology (Oxford). 2021;60(3):1387–91.
Mertens JS, et al. Use of mycophenolate mofetil in patients with severe localized scleroderma resistant or intolerant to methotrexate. Acta Derm Venereol. 2016;96(4):510–3.
Arthur M, et al. Evaluation of the effectiveness and tolerability of mycophenolate mofetil and mycophenolic acid for the treatment of morphea. JAMA Dermatol. 2020;156(5):521–8.
McGaugh S, et al. Janus kinase inhibitors for treatment of morphea and SSc: a literature review. Dermatol Ther. 2022;35(6):e15437.
Aung WW, et al. Immunomodulating role of the JAKs inhibitor tofacitinib in a mouse model of bleomycin-induced scleroderma. J Dermatol Sci. 2021;101(3):174–84.
Damsky W, et al. Jak inhibition prevents bleomycin-induced fibrosis in mice and is effective in patients with morphea. J Invest Dermatol. 2020;140(7):1446–1449 e4.
Kim SR, et al. Treatment of generalized deep morphea and eosinophilic fasciitis with the Janus kinase inhibitor tofacitinib. JAAD Case Rep. 2018;4(5):443–5.
Scheinberg M, et al. Full histological and clinical regression of morphea with tofacitinib. Clin Rheumatol. 2020;39(9):2827–8.
Soh HJ, et al. Challenges in the diagnosis and treatment of disabling pansclerotic morphea of childhood: case-based review. Rheumatol Int. 2019;39(5):933–41.
Distler JH, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56(1):311–22.
Alcantara-Reifs CM, et al. Imatinib treatment of therapy resistant generalized deep morphea. Dermatol Ther. 2015;28(5):271–3.
Coelho-Macias V, et al. Imatinib: a novel treatment approach for generalized morphea. Int J Dermatol. 2014;53(10):1299–302.
Inamo Y, Ochiai T. Successful combination treatment of a patient with progressive juvenile localized scleroderma (morphea) using imatinib, corticosteroids, and methotrexate. Pediatr Dermatol. 2013;30(6):e191–3.
Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol. 2010;63(5):e102–4.
clinicaltrials.gov. Efficacy and safety of imatinib in scleroderma (SCLEROGLIVEC). ClinicalTrials.gov ID NCT00479934. Sponsor: University Hospital, Bordeaux. Information provided by University Hospital, Bordeaux.
Ponsoye M, et al. Treatment with abatacept prevents experimental dermal fibrosis and induces regression of established inflammation-driven fibrosis. Ann Rheum Dis. 2016;75(12):2142–9.
Adeeb F, et al. Early- and late-stage morphea subtypes with deep tissue involvement is treatable with abatacept (Orencia). Semin Arthritis Rheum. 2017;46(6):775–81.
Fage SW, Arvesen KB, Olesen AB. Abatacept improves skin-score and reduces lesions in patients with localized scleroderma: a case series. Acta Derm Venereol. 2018;98(4):465–6.
Li SC, et al. Preliminary evidence on abatacept safety and efficacy in refractory juvenile localized scleroderma. Rheumatology (Oxford). 2021;60(8):3817–25.
Stausbol-Gron B, et al. Abatacept is a promising treatment for patients with disseminated morphea profunda: presentation of two cases. Acta Derm Venereol. 2011;91(6):686–8.
Talia J, et al. A Case of recalcitrant linear morphea responding to subcutaneous abatacept. J Scleroderma Relat Disord. 2021;6(2):194–8.
Kalampokis I, Yi BY, Smidt AC. Abatacept in the treatment of localized scleroderma: a pediatric case series and systematic literature review. Semin Arthritis Rheum. 2020;50(4):645–56.
Kromer C, et al. Response of recalcitrant generalized morphea to intravenous immunoglobulins (IVIg): three cases and a review of the literature. Eur J Dermatol. 2021;31(6):822–9.
Gutierrez D, et al. Eosinophilic fasciitis with concomitant morphea profunda treated with intravenous immunoglobulin. J Clin Rheumatol. 2021;27(8S):S500–1.
Kucukoglu R, Yilmaz Z, Kutlay A. Treatment of recalcitrant generalized morphea with mycophenolate mofetil and intravenous immunoglobulin. Dermatol Ther. 2018;31(5): e12674.
Cantarini L, et al. Intravenous immunoglobulins (IVIG) in SSc: a challenging yet promising future. Immunol Res. 2015;61(3):326–37.
Blank M, et al. The role of intravenous immunoglobulin therapy in mediating skin fibrosis in tight skin mice. Arthritis Rheum. 2002;46(6):1689–90.
Kajii M, et al. Prevention of excessive collagen accumulation by human intravenous immunoglobulin treatment in a murine model of bleomycin-induced scleroderma. Clin Exp Immunol. 2011;163(2):235–41.
Kudo H, et al. Intravenous immunoglobulin treatment recovers the down-regulated levels of Th1 cytokines in the sera and skin of scleroderma patients. J Dermatol Sci. 2013;69(1):77–80.
Johnson BZ, et al. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines. 2020;8(5):101.
Blaise M, et al. Tocilizumab for corticosteroid-refractory immune checkpoint inhibitor-induced generalized morphea. JAMA Dermatol. 2023;159(1):112–4.
Lonowski S, et al. Tocilizumab for refractory morphea in adults: a case series. JAAD Case Rep. 2022;30:27–9.
Lythgoe H, et al. Tocilizumab as a potential therapeutic option for children with severe, refractory juvenile localized scleroderma. Rheumatology (Oxford). 2018;57(2):398–401.
Martini G, et al. Tocilizumab in two children with pansclerotic morphoea: a hopeful therapy for refractory cases? Clin Exp Rheumatol. 2017;35(Suppl 106 (4)):211–3.
Zhang A, Nocton J, Chiu Y. A case of pansclerotic morphea treated with tocilizumab. JAMA Dermatol. 2019;155(3):388–9.
Magro CM, et al. Linear scleroderma “en coup de sabre” with extensive brain involvement-Clinicopathologic correlations and response to anti-Interleukin-6 therapy. Orphanet J Rare Dis. 2019;14(1):110.
Quintarelli L, et al. Unilateral generalised morphea successfully treated with rituximab and mycophenolate mofetil. Clin Exp Rheumatol. 2021;39(6):1449–50.
Chimenti MS, et al. Resolution with rituximab of localized scleroderma occurring during etanercept treatment in a patient with rheumatoid arthritis. Eur J Dermatol. 2013;23(2):273–4.
Traboulsi D, et al. Morphea associated with primary biliary cirrhosis and Waldenstrom macroglobulinemia: response to rituximab. JAAD Case Rep. 2018;4(8):784–7.
clinicaltrials.gov. A protocol based treatment for debilitating fibrosing skin disorders with (anti-CD 20), rituximab, evaluating safety and efficacy.
clinicaltrials.gov. Clinical trial to evaluate efficacy and safety of dupilumab in localized scleroderma (DupiMorph).
Bukiri H, Volkmann ER. Current advances in the treatment of SSc. Curr Opin Pharmacol. 2022;64: 102211.
Wenzel D, et al. Upcoming treatments for morphea. Immun Inflamm Dis. 2021;9(4):1101–45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Not applicable.
Conflict of interest
Not applicable.
Availability of data and materials
Not applicable.
Ethics approval
Not applicable.
Patient consent to participate/publish
Not applicable.
Code availability
Not applicable.
Author contributions
Dr. Teske wrote the entire section on morphea. Dr. Fett wrote the entire section on SSc. Both Drs. Teske and Fett edited the entire manuscript as well as read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teske, N., Fett, N. Recent Advances in Treatment of Systemic Sclerosis and Morphea. Am J Clin Dermatol 25, 213–226 (2024). https://doi.org/10.1007/s40257-023-00831-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-023-00831-2