Abstract
Objective
To assess the effect of activated charcoal on the single-dose pharmacokinetics of mavacamten when administered 2 h or 6 h after mavacamten dosing.
Methods
In this open-label, randomized, parallel-group study, healthy adults were randomized into three groups to receive mavacamten 15 mg alone or mavacamten 15 mg plus activated charcoal 50 g administered either 2 h or 6 h after mavacamten dosing. Pharmacokinetic parameters were derived from plasma concentration–time data using noncompartmental methods.
Results
Of the 45 participants randomized, 37 completed the study. When activated charcoal was administered 2 h after mavacamten dosing, mavacamten absorption and exposure were reduced compared with when mavacamten was administered alone: the area under the concentration–time curve from 0 to 72 h (AUC0–72) and area under the concentration–time curve from time 0 extrapolated to infinity (AUCINF) were reduced by 14% and 34%, respectively. The maximum plasma concentration (Cmax) was also slightly lower when activated charcoal was administered 2 h after mavacamten dosing than with mavacamten alone. Pharmacokinetic profiles were similar for mavacamten alone and mavacamten plus activated charcoal administered 6 h after mavacamten dosing.
Conclusions
Activated charcoal was successful in reducing mavacamten absorption and exposure when administered as soon as possible after identification of a need for adsorption (2 h after mavacamten dosing). No change in exposure was observed when activated charcoal was administered 6 h after mavacamten dosing.
Clinical Trial Registration
NCT05320094
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Currently, there is no antidote for an overdose of mavacamten, a drug approved for the treatment of adults with New York Heart Association class II–III obstructive hypertrophic cardiomyopathy. This study investigated the effects of activated charcoal on mavacamten exposure 2 h or 6 h after mavacamten dosing. |
The results indicate that, when administered as soon as possible after an overdose or accidental ingestion of mavacamten, activated charcoal has the highest potential for benefit in reducing the absorption, and subsequent exposure of mavacamten, suggesting its potential utility in acute overdose management. |
1 Introduction
Mavacamten is the first cardiac myosin inhibitor approved in five continents for the treatment of adults with symptomatic New York Heart Association class II–III obstructive hypertrophic cardiomyopathy (HCM) [1,2,3,4,5,6,7]. Mavacamten targets the underlying pathophysiology of obstructive HCM by selectively and reversibly inhibiting cardiac myosin by binding at its allosteric site [8, 9].
No specific antidote to mavacamten is currently available for use in the event of an overdose. Due to its mechanism of action, mavacamten can reduce left ventricular ejection fraction or cardiac output, which in extreme cases may lead to symptoms of systolic dysfunction [1]. Thus, although no direct relationship between mavacamten exposure and increased risk of heart failure due to systolic dysfunction have been established, an overdose of mavacamten could potentially increase the risk of heart failure. Activated charcoal is routinely used to manage accidental ingestion or overdose of medicines by adsorbing drugs present in the gastrointestinal (GI) tract [10]. In human volunteer studies, activated charcoal has been shown to limit exposure to many different compounds to varying degrees [11]. Mavacamten is rapidly absorbed following oral administration, with an estimated oral bioavailability of at least 85% and time to maximum concentration (Tmax) of 1 h [1], which is increased by 4 h with a high-fat meal [1]. Mavacamten is eliminated primarily via cytochrome P (CYP) 450 enzymes (74% CYP2C19, 18% CYP3A4, and 8% CYP2C9) [1]. It has a biphasic elimination profile with a long mean apparent terminal half-life of 6–9 days in normal CYP2C19 metabolizers and of up to 23 days in poor CYP2C19 metabolizers [1].
The objective of the study was to assess the effect of activated charcoal on the pharmacokinetics (PK) of single-dose mavacamten when administered 2 h or 6 h after mavacamten dosing in healthy participants. A physiologically based pharmacokinetic (PBPK) model was utilized to support understanding and interpretation of the findings from the clinical study, and to provide additional insights.
2 Methods
This was an open-label, randomized, parallel-group study (NCT05320094). Eligible participants were healthy male and female volunteers, aged 18–60 years, with a body mass index between 18 and 30 kg/m2, inclusive. Participants were randomized according to a computer-generated scheme to receive a mavacamten 15 mg capsule, a mavacamten 15 mg capsule plus activated charcoal 50 g administered 2 h after mavacamten dosing, or a mavacamten 15 mg capsule plus activated charcoal 50 g administered 6 h after mavacamten dosing. The 50 g dose of activated charcoal is the standard for treating overdose in adults [10] and was administered with sorbitol 52 g in a 240 mL suspension. Participants provided written informed consent prior to study commencement. The study protocol was approved by the SALUS Institutional Review Board (8482838) on 25 February 2022 and conducted in compliance with all local regulations, the Declaration of Helsinki, and the International Conference on Harmonization Guidelines for Good Clinical Practice.
The primary endpoints in this study were the following mavacamten PK parameters: area under the concentration–time curve from time 0 extrapolated to infinity (AUCINF), area under the concentration–time curve from time 0 to the time of the last quantifiable concentration (AUC0–T), and maximum plasma concentration (Cmax). Area under the concentration–time curve from time 0 to 72 h (AUC0–72) was also analyzed. PK analyses were performed following the methods used by Wang et al. 2014 [12]. Blood samples for PK analysis were collected for up to 45 (± 2) days after study intervention administration. Mavacamten PK parameters were derived from plasma concentration–time data using noncompartmental methods. Analyses were performed on the natural logarithms of AUC0–72, AUCINF, AUC0–T, and Cmax using the ANOVA model with treatment as a fixed effect. These calculations assumed that Cmax and AUCINF were log-normally distributed with inter-participant coefficients of variation of 0.47 and 0.40, respectively.
Approximately 42 participants were to be randomized to study intervention such that approximately 36 participants completed the study. This target number was selected because 12 evaluable participants in each of the three groups would provide an 80% probability that the 90% confidence intervals (CIs) of the geometric mean ratio of Cmax for mavacamten would lie within 70% and 143% of the point estimate and the 90% CIs of the geometric mean ratio of AUCINF for mavacamten would lie within 74% and 136% of the point estimate.
Safety assessments included adverse events (AEs), clinical laboratory tests, vital sign measurements, physical examinations, and 12-lead electrocardiograms.
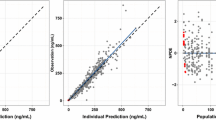
A PBPK model was adapted to include mechanistic absorption using Simcyp v22 M-ADAM by including mavacamten permeability across enterocytes [13]. Solubility-related parameters (mavacamten intrinsic solubility and bile micelle partitioning) were informed by SIVA v4 analysis of in vitro solubility data. A full PBPK model with tissue partition coefficients was informed by rat quantitative whole body autoradiography data. An interaction with charcoal was performed using Simcyp’s excipient binding model by creating a “dummy” compound with charcoal parameters being defined as an excipient with high solubility and low permeability to allow reversible binding to dissolved, unbound mavacamten solely in the intestinal lumen. A high excipient binding constant (1E5 M−1) for mavacamten to charcoal was informed by sensitivity analysis to maximize the interaction between the two substances. This adapted model was leveraged for in-silico crossover studies, after model qualification. Details of PBPK model parameters and performance are in Supplementary Table 1.
3 Results
In total, 45 participants (48% of those screened) were randomized to treatment, and 37 completed the study. Of the eight individuals who did not complete the study, one withdrew, and seven discontinued because of an AE of coronavirus disease 2019 (COVID-19), which was considered unrelated to study treatment. All participants received all planned doses of study treatment except for the participant who withdrew after receiving mavacamten 15 mg on day 1. Baseline characteristics were generally similar across groups, although there were some differences in the distribution of CYP2C19 genotypes/phenotypes (Table 1).
Administration of activated charcoal 2 h after mavacamten dosing resulted in 14% lower mavacamten absorption (determined by AUC0–72) and 34% lower fraction of mavacamten dose absorbed (assessed with AUC0–INF) compared with administration of mavacamten alone (Table 2; Fig. 1A). Inter-participant variability was high, ranging from 41 to 58%. Cmax was not affected significantly by the administration of activated charcoal 2 or 6 h after mavacamten dosing. Cmax of mavacamten was reached at a similar time for all three treatment groups (Fig. 2). Median (minimum–maximum) Tmax was 1.5 (0.5–4.0), 1.5 (1.0–6.1), and 1.0 (0.5–3.0) h for mavacamten 15 mg alone, mavacamten 15 mg plus activated charcoal administered 2 h after mavacamten dosing, and mavacamten 15 mg plus activated charcoal administered 6 h after mavacamten dosing, respectively. Although PK profiles were similar for mavacamten alone and mavacamten plus activated charcoal administered 6 h after mavacamten dosing, differences were noted when activated charcoal was administered 2 h after mavacamten dosing (Table 2; Figs. 1, 2). The half-life (mean [standard deviation]) of mavacamten was approximately 3 days shorter in the group treated with activated charcoal 2 h after mavacamten dosing (5.60 [2.69] days) than among participants who received mavacamten alone (8.64 [6.19] days); however, this reduced half-life was not seen for the group treated with activated charcoal 6 h after mavacamten dosing (8.84 [4.89] days) and may represent the difference in CYP2C19 metabolizer phenotypes.
Geometric LS mean ratios for PK parameters comparing mavacamten 15 mg + activated charcoal administered 2 h after mavacamten dosing and mavacamten 15 mg alone (a) and comparing mavacamten 15 mg + activated charcoal administered 6 h after mavacamten dosing and mavacamten 15 mg alone (b). Vertical lines represent bioequivalence acceptance criteria (0.8–1.25). AUC0–72 area under the concentration–time curve from time 0 to 72 h, AUC0–T area under the concentration–time curve from time 0 to the time of the last quantifiable concentration, AUCINF area under the concentration–time curve from time 0 extrapolated to infinity, CI confidence interval, Cmax maximum plasma concentration, LS least-squares, PK pharmacokinetic
Due to the imbalance in CYP2C19 phenotypes between the control arm (mavacamten alone) and the 2-h charcoal arm, a sensitivity analysis was performed without intermediate metabolizers. The goal of this sensitivity analysis was to assess the relevance of the findings in this study (i.e. to investigate whether the imbalance led to the difference in PK parameters rather than charcoal administration). Due to the ensuing small sample size of the mavacamten alone group and because no effects were observed when activated charcoal was administered 6 h after mavacamten, the 2 groups were combined. This resulted in a reference group which comprised 12 (63%) normal metabolizers and 7 (37%) rapid metabolizers while the 2-h activated charcoal arm comprised 10 (77%) normal metabolizers and 3 (23%) rapid metabolizers. The results from the sensitivity analysis confirmed the current findings with AUC0–72 and AUC0-inf 25% and 30% lower in the 2-h activated charcoal arm compared with the reference arm, respectively.
There were no deaths or serious AEs. Twenty participants (44%) experienced 25 treatment-emergent AEs (TEAEs), all of which were mild and had resolved by the end of the study. The most common TEAEs were COVID-19 (n = 7), headache (n = 6), viral infection (n = 2), and vomiting (n = 2). Nine participants reported TEAEs that were considered to be related to mavacamten treatment, including six events of headache, one of syncope, one of diarrhea, and one of genital paresthesia. Two events of vomiting and one of nausea were considered to be related to treatment with both mavacamten and activated charcoal. There were no clinically significant laboratory abnormalities, no TEAEs related to laboratory evaluations, and no AEs reported based on electrocardiogram abnormalities.
Simulations from the PBPK model, which used a crossover approach in-silico, were able to recover the observed changes in mavacamten AUC and Cmax with administration of charcoal at 2 and 6 h post-mavacamten dose (Supplementary Table 2). The model suggested that in a fasted state, the fraction of the dose already absorbed was about 65% by 2 h and near complete by 6 h, and that charcoal administration would be expected to adsorb any, and all unabsorbed mavacamten. The model was then used to assess the relative impact of administering charcoal at different times relative to mavacamten Tmax. The model confirmed the clinical findings, and supported the expectation that charcoal administration is more effective at removing mavacamten when administered early in the absorption process, ideally around or before mavacamten Tmax.
4 Discussion
Results from this study in healthy participants indicate that activated charcoal may be useful in the management of mavacamten overdose or accidental dosing. Activated charcoal administered 2 h after mavacamten dosing reduced the overall fraction of the mavacamten dose absorbed by 34%. The limited effects of activated charcoal administration on Tmax and Cmax were consistent with expectations because the mavacamten Tmax was achieved in most participants at or prior to the 2-h time point. Nevertheless, this study demonstrated that administration of activated charcoal could have a beneficial effect, even when administered slightly after Tmax of mavacamten (i.e., 2 h after mavacamten dosing). Due to the unfortunate imbalance in CYP2C19 phenotypes between groups, with an overall higher number of more efficient metabolizers in the 2-h post dose group relative to the other two groups, a sensitivity analysis was performed to provide confidence in the interpretation of the clinical findings. In addition, the PBPK model was used, to simulate in silico trials using a crossover design, to overcome the limitations of a parallel clinical study design necessitated by the long half-life of mavacamten. This assessment predicted an effect of similar magnitude to the clinical data observed at both time points, i.e., 2 and 6 h post-dose, giving reassurance that the clinical study provided valuable information, despite the imbalance in CYP2C19 phenotypes between treatment arms. Furthermore, the PBPK model also provided additional insights, i.e., activated charcoal could be expected to adsorb any unabsorbed mavacamten in the GI tract, and by Tmax or slightly after (based on the present results and on previous studies, Tmax is approximately 1–2 h when mavacamten is dosed in a fasted state and delayed to ~ 4 h in a fed state) only about 35% remains unabsorbed. By 3–4 h after Tmax, absorption is near complete, providing useful context for the clinical findings, where mavacamten was dosed in a fasted state. Therefore, administration of activated charcoal as early as possible relative to mavacamten dosing would be expected to provide maximal potential benefit, with no expected benefit once absorption of mavacamten is complete.
Although activated charcoal primarily reduces systemic exposure to drugs by adsorbing the drug in the gut and preventing absorption, elimination is also affected for some compounds [14]. The reduction in mavacamten half-life by approximately 3 days in participants who received activated charcoal 2 h after mavacamten dosing compared with those who received mavacamten alone may indicate an augmentation of elimination of mavacamten by the activated charcoal. Activated charcoal may disrupt the recycling of mavacamten between the systemic circulation and the GI tract (enteroenteric recycling), resulting in increased elimination via feces [14]. However, it is important not to overinterpret the observed difference in half-life, due to the imbalance in CYP2C19 genotypes/phenotypes between the groups receiving mavacamten alone and mavacamten plus activated charcoal 2 h after mavacamten.
Activated charcoal administration times of 2 h and 6 h were chosen to mimic potential real-life situations of overdose management while generally bracketing the absorption profile of mavacamten. Indeed, the 2-h and 6-h time points correspond approximately to the Tmax of mavacamten in the fasted state (1–2 h) and the fed state (4–6 h), respectively [1]. It is worth noting that mavacamten absorption is expected to be nearly complete 6 h after administration. The 2-h time point for charcoal administration was selected to allow for a realistic scenario between identification of a potential overdose, and timing of charcoal administration because this is the likely time of arrival at the emergency department after an overdose, assuming that there is a time lag between ingestion, identification of the event, and access to activated charcoal, either at home or at the emergency room (patients are rarely admitted within 1 h of ingestion) [10]. It would certainly have been possible to design a study with a 1-h lag, which would likely result in a greater fraction of the dose absorbed in the study, but 1 h post-dose may be unrealistic to identify an overdose and administer charcoal in the real world, unless charcoal was available at home, and identification was less than 1 h post-dose.
The effectiveness of activated charcoal in reducing the overall fraction absorbed of the mavacamten dose builds confidence in the use of mavacamten in clinical practice. The model suggested that charcoal adsorbs nearly all mavacamten remaining to be absorbed in the gut. Thus, if administered as soon as possible after mavacamten dosing, activated charcoal ingestion can result in substantial reduction in exposure. In the presence of food, since the Tmax is delayed, the potential for charcoal to adsorb mavacamten is prolonged, consistent with the prolongation of the absorption period. Thus, in the fed state, charcoal administration is expected to meaningfully reduce absorption of mavacamten beyond 2 h, as Tmax is 4–6 h in the fed state. Near immediate administration, as soon as possible after identification of accidental ingestion of mavacamten, is recommended for maximal benefit of charcoal administration to reduce mavacamten exposure. However, the study did not specifically explore the effectiveness of activated charcoal in an overdose situation, and there may be additional factors to be considered in terms of dose and timing in such an emergency.
In conclusion, administration of activated charcoal 2 h after mavacamten dosing reduced mavacamten absorption and exposure, whereas administration of activated charcoal 6 h after mavacamten dosing did not affect mavacamten absorption/exposure. Activated charcoal may be useful in the management of mavacamten overdose, and the effect is most pronounced when given as soon as possible after ingestion, ideally prior to Tmax, which is delayed in the fed state.
References
CAMZYOS® (mavacamten). US prescribing information. Princeton: Bristol Myers Squibb Company; 2023.
CAMZYOS® (mavacamtenum). Professional information, Swissmedic: Bristol Myers Squibb SA; 2023.
CAMZYOS® (mavacamteno). Brazilian bula profissional: São Paulo, Brazil: Farmacêutica LTDA; 2023.
CAMZYOS® (mavacamten). Korean prescribing information: Bristol Myers Squibb; 2023.
CAMZYOS® (mavacamten). Macao prescribing information: Bristol Myers Squibb; 2023.
CAMZYOS™ (mavacamten). Canadian product monograph: Montreal, Canada: Bristol Myers Squibb Canada; 2022.
CAMZYOS® (mavacamten). Australian product information: Victoria, Australia: Bristol Myers Squibb Australia Pty Ltd; 2022.
Tuohy CV, Kaul S, Song HK, Nazer B, Heitner SB. Hypertrophic cardiomyopathy: the future of treatment. Eur J Heart Fail. 2020;22(2):228–40.
Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, et al. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci USA. 2018;115(35):E8143–52.
Megarbane B, Oberlin M, Alvarez JC, Balen F, Beaune S, Bedry R, et al. Management of pharmaceutical and recreational drug poisoning. Ann Intensive Care. 2020;10(1):157.
Jurgens G, Hoegberg LC, Graudal NA. The effect of activated charcoal on drug exposure in healthy volunteers: a meta-analysis. Clin Pharmacol Ther. 2009;85(5):501–5.
Wang X, Mondal S, Wang J, Tirucherai G, Zhang D, Boyd RA, et al. Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am J Cardiovasc Drugs. 2014;14(2):147–54.
Chiang M, Sychterz C, Perera V, Merali S, Palmisano M, Templeton IE, et al. Physiologically based pharmacokinetic modeling and simulation of mavacamten exposure with drug-drug interactions from CYP inducers and inhibitors by CYP2C19 phenotype. Clin Pharmacol Ther. 2023;114(4):922–32.
Zellner T, Prasa D, Farber E, Hoffmann-Walbeck P, Genser D, Eyer F. The use of activated charcoal to treat intoxications. Dtsch Arztebl Int. 2019;116(18):311–7.
Acknowledgement
The authors thank all participants in this study, as well as the clinical study teams for their contributions. Medical writing and editorial assistance was provided by Nicolas Bertheleme Ph.D. of Oxford PharmaGenesis, Oxford, UK, funded by Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Bristol Myers Squibb.
Conflict of interest
S.M., M.C., C.S., L.C., T.S., Y.X., M.A., and B.M. are employees of Bristol Myers Squibb and own stocks in Bristol Myers Squibb; V.P. was an employee of Bristol Myers Squibb at the time of this work; and A.Z. is an employee of Cytel, which receives fees for services provided to Bristol Myers Squibb.
Author contributions
Samira Merali, Manting Chiang, Alice Zhao, Bindu Murthy, and Vidya Perera contributed to the study conception and design. Caroline Sychterz, Longfei Chao, Tara Simmons, and Yiru Xu contributed to data acquisition. Caroline Sychterz, Longfei Chao, and Tara Simmons contributed to the analysis of the data. Caroline Sychterz, Samira Merali, Massimo Attanasio, and Vidya Perera contributed to data interpretation. All authors contributed and critically reviewed the first draft, and all authors read and approved the final version of the manuscript.
Data availability
Bristol Myers Squibb’s policy on data sharing is available online and is located at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
Ethics approval
The study protocol was approved by SALUS Institutional Review Board (8482838) on 25 February 2022 and conducted in compliance with all local regulations, the Declaration of Helsinki, and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Consent to participate
Participants provided written informed consent prior to study commencement.
Code availability
Not applicable.
Consent for publication
Not applicable.
Additional information
Vidya Perera: Affiliation at time of study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Merali, S., Chiang, M., Sychterz, C. et al. Effect of Activated Charcoal on Mavacamten Pharmacokinetics in Healthy Participants. Am J Cardiovasc Drugs 24, 569–575 (2024). https://doi.org/10.1007/s40256-024-00659-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-024-00659-z