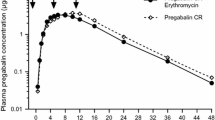
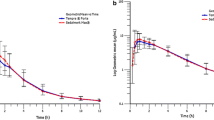
Abstract
Purpose
Activated charcoal is known to adsorb a variety of drugs concomitantly administered and reduce their intestinal absorption, and separating the dosing is considered a practical approach to avoid this drug interaction. The aim of the present study was to develop and validate a simple method to estimate the sufficient dosing interval to avoid drug interaction using the pharmacokinetic profile of the subject drugs administered alone and the amplitude of interaction upon simultaneous administration with activated charcoal.
Methods
For each subject drug, the pharmacokinetic profile and the amplitude of interaction, as assessed by AUCR (the ratio of area under the plasma concentration-time curve (AUC) in the presence of activated charcoal to that in its absence), were collected from previous reports. The AUCR value was estimated based on the compartment model under the assumption that the subject drug in the first gastrointestinal compartment is immediately adsorbed to a certain extent upon the administration of activated charcoal. The estimated AUCR (AUCRe) for each drug with certain dosing interval was compared with the respective AUCR value reported previously (AUCRobs).
Results
Among twenty concentration profiles for 14 subject drugs obtained from previous reports, 15 AUCRe values fell in the range of 80–120% of the respective AUCRobs values.
Conclusion
The developed method enabled estimation of the amplitude of DDI by activated charcoal administered in a certain dosing interval, whereas overestimation of AUCRe was observed for drugs that undergo extensive enterohepatic circulation.



Similar content being viewed by others
Data availability
Data sharing not applicable to this study as no datasets were generated during current study. All data analyzed during this study are included in this published (the reference) articles.
Change history
16 September 2020
Figure 3 image was inadvertently removed from the original article. The original article has been corrected.
Abbreviations
- AIC:
-
Akaike’s information criterion
- AUC:
-
Area under the plasma concentration-time curve
- AUCR:
-
AUC ratio (the ratio of AUC in the presence of activated charcoal to that in the absence of activated charcoal)
- AUCRobs :
-
Observed AUCR
- AUCRe :
-
Estimated AUCR
- D:
-
Dose
- DDI:
-
Drug-drug interaction
- k12 :
-
Rate constant from central compartment to peripheral compartment
- k21 :
-
Rate constant from peripheral compartment to central compartment
- ke :
-
Elimination rate constant
- kG1 :
-
Transfer rate constant from GI1 compartment
- kG2 :
-
Transfer rate constant from GI2 compartment to systemic compartment
- tlag :
-
Lag time
- tmax :
-
Time to reach maximum plasma concentration
- Vd :
-
Volume of distribution
References
Welling PG (1984) Interactions affecting drug absorption. Clin Pharmacokinet 9(5):404–434. https://doi.org/10.2165/00003088-198409050-00002
Miyata K, Ohtani H, Tsujimoto M, Sawada Y (2007) Antacid interaction with new quinolones: dose regimen recommendations based on pharmacokinetic modeling of clinical data for ciprofloxacin, gatifloxacin and norfloxacin and metal cations. Int J Clin Pharmacol Ther 45(1):63–70. https://doi.org/10.5414/cpp45063
Asai M, Kumakura S, Kikuchi (2019) Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Ren Fail 41:47–56. https://doi.org/10.1080/0886022X.2018.1561376
Niwa T, Ise M (1994) Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med 124:96–104
Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) (2009) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4:1551–1558. https://doi.org/10.2215/CJN.03980609
Schulman G, Agarwal R, Acharya M, Berl T, Blumenthal S, Kopyt N (2006) A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis 47:565–577. https://doi.org/10.1053/j.ajkd.2005.12.036
Sanaka T (2009) The points to note in carbonaceous adsorptive agent. Kidney Dial 67:450–456 [Japanese]
Koshikawa S, Koide K, Yamane (1992) The effect of AST-120 on delaying initiation of dialysis therapy in end-stage renal disease. Kidney Dial 32:783–794 [Japanese]
Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M (2015) Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol 26:1732–1746. https://doi.org/10.1681/ASN.2014010042
Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Shimizu M, Kikuchi M, Shobu Y (2018) Risk factors for progression of chronic kidney disease in the EPPIC trials and the effect of AST-120. Clin Exp Nephrol 22:299–308. https://doi.org/10.1007/s10157-017-1447-0
Olson KR (2010) Activated charcoal for acute poisoning: one toxicologist’s journey. J Med Toxicol 6(2):190–198. https://doi.org/10.1007/s13181-010-0046-1
Laine K, Kivistö KT, Laakso I, Neuvonen PJ (1997) Prevention of amlodipine absorption by activated charcoal: effect of delay in charcoal administration. Br J Clin Pharmacol 43(1):29–33. https://doi.org/10.1111/j.1365-2125.1997.tb00029.x
Yamaoka K, Tanigawara Y, Nakagawa T, Uno T (1981) A pharmacokinetics analysis program (multi) for microcomputer. Aust J Pharm 4(11):879–885. https://doi.org/10.1248/bpb1978.4.879
Locatelli I, Mrhar A, Bogataj M (2009) Gastric emptying of pellets under fasting conditions: a mathematical model. Pharm Res 26(7):1607–1617. https://doi.org/10.1007/s11095-009-9869-3
Yeates PJ, Thomas SH (2000) Effectiveness of delayed activated charcoal administration in simulated paracetamol (acetaminophen) overdose. Br J Clin Pharmacol 49(1):11–14. https://doi.org/10.1046/j.1365-2125.2000.00107.x
Christophersen AB, Levin D, Hoegberg LC, Angelo HR, Kampmann JP (2002) Activated charcoal alone or after gastric lavage: a simulated large paracetamol intoxication. Br J Clin Pharmacol 53(3):312–317. https://doi.org/10.1046/j.0306-5251.2001.01568.x
Kivistö KT, Neuvonen PJ (1991) Effect of activated charcoal on the absorption of amiodarone. Hum Exp Toxicol 10(5):327–329. https://doi.org/10.1177/096032719101000505
Meng X, Mojaverian P, Doedée M, Lin E, Weinryb I, Chiang ST, Kowey PR (2001) Bioavailability of amiodarone tablets administered with and without food in healthy subjects. Am J Cardiol 87(4):432–435. https://doi.org/10.1016/s0002-9149(00)01396-5
Liu Y, Jia J, Liu G, Li S, Lu C, Liu Y, Yu C (2009) Pharmacokinetics and bioequivalence evaluation of two formulations of 10-mg amlodipine besylate: an open-label, single-dose, randomized, two-way crossover study in healthy Chinese male volunteers. Clin Ther 31(4):777–783. https://doi.org/10.1016/j.clinthera.2009.04.013
Wang X, Mondal S, Wang J, Tirucherai G, Zhang D, Boyd RA, Frost C (2014) Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am J Cardiovasc Drugs 14(2):147–154. https://doi.org/10.1007/s40256-013-0055-y
Neuvonen PJ, Elonen E (1980) Effect of activated charcoal on absorption and elimination of phenobarbitone, carbamazepine and phenylbutazone in man. Eur J Clin Pharmacol 17(1):51–57. https://doi.org/10.1007/BF00561677
Tothfalusl L, Speidl S, Endrenyi L (2007) Exposure-response analysis reveals that clinically important toxicity difference can exist between bioequivalent carbamazepine tablets. Br J Clin Pharmacol 65(1):110–122. https://doi.org/10.1111/j.1365-2125.2007.02984.x
Neuvonen PJ, Elfving SM, Elonen E (1978) Reduction of absorption of digoxin, phenytoin and aspirin by activated charcoal in man. Eur J Clin Pharmacol 13(3):213–218. https://doi.org/10.1007/BF00609985
Ragueneau I, Poirier JM, Radembino N, Sao AB, Funck-Brentano C, Jaillon P (1999) Pharmacokinetic and pharmacodynamic drug interactions between digoxin and macrogol 4000, a laxative polymer, in healthy volunteers. Br J Clin Pharmacol 48(3):453–456. https://doi.org/10.1046/j.1365-2125.1999.00025.x
Lapatto-Reiniluoto O, Kivistö KT, Neuvonen PJ (1999) Effect of activated charcoal alone or given after gastric lavage in reducing the absorption of diazepam, ibuprofen and citalopram. Br J Clin Pharmacol 48(2):148–153. https://doi.org/10.1046/j.1365-2125.1999.00995.x
Dewland PM, Reader S, Berry P (2009) Bioavailability of ibuprofen following oral administration of standard ibuprofen, sodium ibuprofen or ibuprofen acid incorporating poloxamer in healthy volunteers. BMC Clin Pharmacol 9:19. https://doi.org/10.1186/1472-6904-9-19
Keränen T, Sorri A, Moilanen E, Ylitalo P (2010) Effects of charcoal on the absorption and elimination of the antiepileptic drugs lamotrigine and oxcarbazepine. Arzneimittelforschung. 60(7):421–426. https://doi.org/10.1055/s-0031-1296306
Sharma C, Dubey R, Kumar H, Saha N (2005) Food reduces the bioavailability of lamotrigine. Indian J Med Res 121(5):659–664
Dalmora SL, Sangoi MS, Nogueira DR, D’Avila FB, Moreno RA, Sverdloff CE, Oliveira RA, Borges NC (2010) Determination of phenobarbital in human plasma by a specific liquid chromatography method: application to a bioequivalence study. Quim Nova 33(1):124–129. https://doi.org/10.1590/S0100-40422010000100023
Laine K, Kivistö KT, Ojala-Karlsson P, Neuvonen PJ (1997) Effect of activated charcoal on the pharmacokinetics of pholcodine, with special reference to delayed charcoal ingestion. Ther Drug Monit 19(1):46–50
Lapatto-Reiniluoto O, Kivistö KT, Neuvonen PJ (2000) Gastric decontamination performed 5 min after the ingestion of temazepam, verapamil and moclobemide: charcoal is superior to lavage. Br J Clin Pharmacol 49(3):274–278. https://doi.org/10.1046/j.1365-2125.2000.00138.x
Lapatto-Reiniluoto O, Kivistö KT, Neuvonen PJ (2000) Efficacy of activated charcoal versus gastric lavage half an hour after ingestion of moclobemide, temazepam, and verapamil. Eur J Clin Pharmacol 56(4):285–288. https://doi.org/10.1007/s002280000139
Hanff LM, Rutten WJ (1996) Pharmacokinetic aspects of rectal formulations of temazepam. Pharm World Scl 18(3):114–119. https://doi.org/10.1007/BF00417760
Neuvonen PJ, Vartiainen M, Tokola O (1983) Comparison of activated charcoal and ipecac syrup in prevention of drug absorption. Eur J Clin Pharmacol 24(4):557–562. https://doi.org/10.1007/BF00609903
Potgieter MA, Pretorius SG, Jacobs YL, Venter C, Venter JL, Geisser P (2007) Effect of an oral iron(III)-hydroxide polymaltose complex on tetracycline pharmacokinetics in patients with iron deficiency anemia. Arzneimittelforschung. 57(6a):385–391. https://doi.org/10.1055/s-0031-1296687
John DN, Fort S, Lewis MJ, Luscombe DK (1992) Pharmacokinetics and pharmacodynamics of verapamil following sublingual and oral administration to healthy volunteers. Br J Clin Pharmacol 33(16):623–627. https://doi.org/10.1111/j.1365-2125.1992.tb04091.x
Berg MJ, Berlinger WG, Goldberg MJ, Spector R, Johnson GF (1982) Acceleration of the body clearance of phenobarbital by oral activated charcoal. N Engl J Med 307(11):642–644. https://doi.org/10.1056/NEJM198209093071102
Author information
Authors and Affiliations
Contributions
Conceived of designed study: Ohtani, Imaoka, and Akiyoshi
Performed research/analyzed data: Ohtani, Nakamura, Imaoka, and Akiyoshi
Wrote the paper: Ohtani, Nakamura, Imaoka, and Akiyoshi
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article was revised: Figure 3 image was inadvertently removed from the original article.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Ohtani, H., Nakamura, K., Imaoka, A. et al. Novel method to estimate the appropriate dosing interval for activated charcoal to avoid interaction with other drugs. Eur J Clin Pharmacol 76, 1529–1536 (2020). https://doi.org/10.1007/s00228-020-02931-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02931-y