Abstract
Among novel nanostructured materials, transition metal chalcogenides (i.e., sulfides and selenides) emerged as promising candidates due to their unique electrochemical properties. The following study presents a facile synthesis approach of Bi2S3 nanostructures using solvent mixtures of ethanol and water with different volume ratios and ammonium sulfide as a sulfur precursor. The resultant bismuth sulfides were characterized by field-emission scanning electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and nitrogen sorption at 77 K. The adjustment of the solvent mixture revealed the possibility of customizing the crystalline structure from amorphous to fully crystalline, as well as the morphology of the Bi2S3, which subsequently influenced on their electrochemical properties. Bi2S3 synthesized in a solvent mixture of ethanol-to-water volume ratio 1:2 (Bi2S3-EW12) exhibited almost fully crystalline structure and nanoplatelet-like morphology, which translated to the best electrochemical performance. Bi2S3-EW12 achieved specific capacity of 748 C g−1 in an aqueous 6 mol L−1 KOH electrolyte and maintained the highest capacity value at a large current density of 20 A g−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the recent past, the development of the new electrode materials and electrochemical energy storage systems has flourished intensively. Electrochemical capacitors, which deliver a high amount of energy in short period of time, operate using electrodes mainly based on the carbon materials. Activated carbons, carbon nanotubes and nanofibers, graphene oxides, and reduced graphene oxides are considered for electrochemical capacitor applications due to the high specific surface area and relatively low resistivity [1,2,3,4,5,6,7,8]. Besides carbon materials, transition metal oxides and/or hydroxides [9, 10] seem to be attractive electrode materials as pseudocapacitive material which can generate high capacitance values derived from reversible redox reactions. Additionally, the composites based on carbon and pseudocapacitive materials combine the features of both components [11,12,13] for a new-generation energy storage and conversion systems, which include Na-ion batteries, K-ion batteries, and hybrid capacitors. Moreover, the advanced materials often combine features characteristic of a supercapacitor (capacitor-like) as well as a battery (battery-like) simultaneously. For this reason, the novel type of energy storage devices was proposed, called supercapatteries, which take advantages of both faradaic and capacitive charge storage mechanisms [14].
Nowadays, the low-dimensional metal sulfides, including iron sulfides, cobalt sulfides, tin sulfides, and bismuth sulfides [15,16,17,18,19,20,21], are receiving extensive attention owing to their exceptional properties and a great potential in the wide range of various applications. Due to the semiconductive properties, metal sulfides are considered as suitable material in optoelectronic devices [22], sensors [23], photocatalysis [24, 25], and energy storage devices [26, 27]. According to the literature, Bi2S3 is intensively studied as electrode material for supercapacitors [24, 26, 28,29,30] as well as for lithium-ion batteries [31,32,33]. The Bi2S3 nanoflowers prepared by Liu et al. [28] were characterized by the capacitance value of 233 F g−1 at 1 A g−1. Moreover, Liang et al. [29] used Bi2S3 as electrode active material in supercapacitor, achieving specific capacitance of 270 F g−1 at a current load of 1 A g−1. To improve the supercapacitor performance, the Bi2S3 nanorods and graphene composite were synthesized by Vadivel et al. [30], resulting in a binary material which exhibited a maximum specific capacitance of 290 F g−1 at a current density of 1 A g−1. Promising electrochemical results were obtained for lithium-ion batteries which used electrodes based on Bi2S3 and its composites with carbon materials. Yue et al. [32] and Kumari et al. [33] prepared nanostructural Bi2S3 and used as anode materials achieving discharge capacities of 686 mA h g−1 at 0.2 A g−1 and 532 mA h g−1 at 125 °C, respectively. Furthermore, Chai et al. [31] synthesized Bi2S3/C nanorods which used as anode material were characterized by the specific discharge capacity of 765 mA h g−1 at 0.1 A g−1.
Metal sulfides exhibit hybrid properties combining battery and supercapacitor types of chemistry which is very desirable in case of supercapatteries [34]. Furthermore, bismuth sulfide as a semiconductor with a direct band gap (Eg = 1.3 eV) has aroused extensive interest in the field of electrochemical applications [25].
Among various synthesis techniques of nanostructured bismuth sulfide, the electrochemical deposition [35], sonochemical techniques [36], or microwave irradiation [37] can be distinguished. Nowadays, the commonly used technique for Bi2S3 synthesis is hydrothermal (solvothermal) treatment [28, 38] which requires additional reagents such as thiourea, l-cysteine, thioacetamide, or sulfur sublimed at high temperature [30]. The further disadvantages of mentioned methods are impurities in the final product and high economic cost.
In the following paper, we proposed a facile chemical precipitation of Bi2S3 using solvent mixtures of ethanol and water with different volume ratio. It was found that a solvent mixture was a factor influencing Bi2S3 crystalline structure and morphology which determined their electrochemical performance. The electrochemical measurements exhibited that almost fully crystalline structure and nanoplatelet-like morphology of Bi2S3 led to the best electrochemical properties. The outstanding specific capacity of 748 C g−1 at 0.5 A g−1 was obtained by Bi2S3-EW12 when a solvent mixture of ethanol-to-water volume ratio was 1–2. Moreover, the highest capacity of Bi2S3-EW12 was maintained even at the highest scan rate and current regime.
Experimental section
Materials synthesis
Bismuth sulfides were synthesized using a facile chemical precipitation at 75 °C [26]. The appropriate amount of bismuth nitrate [Bi(NO3)3∙6H2O, > 98%, Sigma-Aldrich] was dissolved in 200 mL of the ethanol and Milli-Q distilled water mixture with various volume ratios. The volume ratios of the ethanol–distilled water mixtures were 1:0, 2:1, 1:1, 1:2, and 0:1. The solutions were adjusted to a set temperature of 75 °C under vigorous mechanical stirring. Subsequently, the water solution of ammonium sulfide [(NH4)2S, 20 wt% in H2O, Sigma-Aldrich] was slowly dropped until precipitation of bismuth sulfide was complete. The resulting bismuth sulfides were washed several times with the appropriate mixture of ethanol and distilled water followed by centrifugation until the neutral pH of the solution. Next, as-prepared materials were dried under a vacuum at 60 °C for 24 h. The samples of Bi2S3 were labeled according to the used volume ratio of ethanol (E) and distilled water (W): Bi2S3-EW10 for 1:0 ratio, Bi2S3-EW21 for 2:1 ratio, Bi2S3-EW11 for 1:1 ratio, Bi2S3-EW12 for 1:2 ratio, and Bi2S3-EW01 for 0:1 ratio.
Characterization
The porous structure of bismuth sulfides was determined by N2 sorption at 77 K using a NOVA 2000 gas sorption analyzer (Quantachrome). Prior to measurements, the sample was outgassed at 80 °C. The specific surface area (SBET) was calculated from the Brunauer–Emmett–Teller (BET) equation. The amount of nitrogen adsorbed at a relative pressure of p/p0 = 0.96 was employed to determine the total pore volume (VT). The micropore volume (VDR) was estimated from the Dubinin-Radushkevich equation. The mesopore volume (Vmes) was determined as the difference between the total pore volume and the micropore volume. The crystalline forms of the synthesized materials were studied by X-ray diffraction analyzer (XRD, Ultima IV, Rigaku) equipped with a 2 kW X-ray tube using Cu Kα2 radiation (λ = 1.54056∙10–10 m). The morphology of bismuth sulfides was analyzed by field-emission scanning electron microscopy (FESEM, Merlin Zeiss) with an accelerating voltage of 3 kV. The chemical composition of resultant bismuth sulfides was determined by X-ray photoelectron spectroscopy (XPS) analysis using a PHI 5000 VersaProbe (ULVAC-PHI) spectrometer.
Electrochemical measurements
The electrodes were composed of 80 wt% of bismuth sulfide as an active electrode material, 10 wt% of polyvinylidene fluoride (PVDF) as a binder, and 10 wt% of carbon black as a percolator. The electrodes were pressed in the form of a pellet with a geometric surface area of 0.9 cm2. The measurements were performed in a three-electrode Swagelok setup using 6 mol L−1 KOH aqueous solution as an electrolyte and gold current collectors to prevent from the corrosion. Electrochemical measurements were conducted with a potentiostat–galvanostat VSP Biologic in a voltage range from − 1.1 to 0 V versus Hg|HgO reference electrode. The electrochemical properties of Bi2S3 samples were determined by cyclic voltammetry at a voltage scan rate from 1 to 100 mV s−1 and galvanostatic charge–discharge at current densities in the range of 0.5–20 A g−1. Additionally, electrochemical impedance spectroscopy (EIS) measurements under an open-circuit potential in the frequency range of 200–10 mHz were performed. The specific capacity was expressed in Coulombs per mass of active material in one electrode. The specific capacity values (Qs/C g−1) were calculated from the cyclic voltammetry curves and galvanostatic discharge profiles using Eqs. (1) and (2), respectively:
where I is the current (A), Δt is the discharge time (s), ν is the scan rate (V s−1), and mel is the mass of the active material in the electrode (g).
Results and discussion
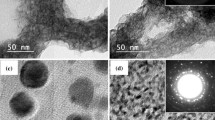
According to the FESEM observations, the morphology of bismuth sulfides changes depending on the ethanol–water volume ratios in the synthesis mixture, Fig. 1. The aggregates of the Bi2S3 spherical nanoparticles were obtained when only ethanol was present as a solvent in the synthesis mixture, Fig. 1a. An addition of water to the ethanol in a volume ratio of 2:1 resulted in a heterogeneous morphology of nanoparticle aggregates and hexagonal small plates, indicating that solvent mixture may influence the crystallinity of Bi2S3, Fig. 1b. When the solvent mixture contains ethanol and water in the same volume ratio (1:1), the large lamellar platelets of Bi2S3 are observed, Fig. 1c. With decreasing contribution of ethanol (1:2 v/v), bismuth sulfide forms mainly large hexagonal platelets with a diameter below 300 nm, along with small, spherical nanoparticles, Fig. 1d. Furthermore, when the exclusive solvent used for the Bi2S3 synthesis is water, the nanorod-like morphology is favored, Fig. 1e. The observed changes in the morphology can be related to the preferential growth of bismuth sulfides crystals along one direction due to the inherent Bi–S chain-type structure and high diffusion rate of ions in water-only environment [39]. However, Bi2S3 nanostructures with smaller aspect ratio were obtained in mixed solvents with an addition of ethanol. Possible growth mechanism of one-dimensional structures can be explained due to the presence of a less polar ethanol, which acts as a capping agent and hinders the anisotropic growth of the initially formed (BiS2)− nuclei, which undergoes decomposition to generate spherical and platelet Bi2S3 structures [40, 41].
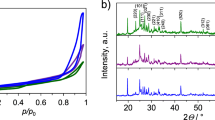
The various morphologies of Bi2S3 obtained in mixtures of a different volume ratio of two solvents are related to their intrinsic structural properties. Bismuth sulfides synthesized via proposed one-pot precipitation method in the presence of different solvents exhibited different crystalline form, from the amorphous to clearly defined crystalline phases. The XRD patterns of all as-prepared Bi2S3 are presented in Fig. 2. No impurities were noticed in the materials synthesized via proposed precipitation method with ammonium sulfide. Only low-intensity peaks corresponding to Bi2O3 phase were observed which proves predominant phase of Bi2S3. The aggregated nanoparticles of the Bi2S3 obtained with ethanol as only solvent presented an amorphous phase without any characteristic peaks of crystalline structure. However, introduction of higher water-to-ethanol volume ratios leads to the transition from the amorphous phase to the orthorhombic phase of Bi2S3 (JCPDS 65-2435), indicating strong influence of the solvent mixture composition on the metal sulfide crystalline structure. The increasing contribution of water results at first in an appearance of the crystalline (130) plane at 2θ of 27.8°, followed by the growth of fully orthorhombic nanorod structure in a water-only environment [40]. The observed phenomenon indicates that the polarity of the solvent mixture plays a crucial role in the bismuth sulfide crystal growth. The lower polarity of ethanol inhibits the growth of long Bi2S3 nanorods, resulting in a low aspect ratio of nanoparticles [42].
Porous structure of bismuth sulfides synthesized with different solvents’ volume ratios was measured by N2 sorption at 77 K. The hysteresis loops revealed the mesoporous nature of Bi2S3, regardless of the solvent mixture during chemical precipitation, Fig. 3. All prepared bismuth sulfides were characterized by the BET surface area in the range from 6 to 32 m2 g−1, proving the poorly developed character of the investigated materials. The total pore volume of Bi2S3 varied from 0.017 to 0.104 cm3 g−1 and the mesopore volume from 0.014 to 0.085 cm3 g−1, Table 1. Among all Bi2S3 samples, the most developed textural was registered for Bi2S3-EW10, synthesized in ethanol due to its morphology of aggregated nanoparticles and amorphous phase, Fig. 3. Addition of distilled water to the ethanol led to the decrease of textural parameters of bismuth sulfides.
The surface chemical composition of the bismuth sulfides obtained in the different solvent were characterized by XPS. Based on the survey spectra (Fig. 4a), no peaks for other elements except Bi and S were detected, indicating the high purity of the obtained products. The peak positions for Bi and S are in good agreement with those reported in the literature [43,44,45]. The high-resolution XPS spectra of Bi 4f and S 2p were explored to determine the chemical state of bismuth sulfides, Fig. 4 b-f. The two strong signals at 158.9 and 164.2 eV can be attributed to Bi 4f7/2 and Bi 4f5/2, respectively. It was reported that in the sample Bi2S3-EW11 and Bi2S3-EW01, two extra peaks at energy binding of 164.7 and 159.4 eV were observed, which can be attributed for appearing bismuth oxide on the surface of Bi2S3.
The electrochemical performance of various Bi2S3 nanostructures in 6 mol L−1 KOH aqueous electrolyte was investigated by cyclic voltammetry and galvanostatic charge–discharge measurements. The CV curves of at various scan rates reveal visible redox peaks, indicating the intense faradaic response of the Bi2S3 nanostructures. The redox peaks at − 0.65 V and − 0.45 V are attributed to the reaction of Bi2S3 with OH− ions, as well as they originate from the change in the bismuth valence state. The faradaic reaction are described according to the equation:
The cathodic peak at − 0.85 V represents the adsorption of hydrogen in the lamellar structure of Bi2S3, while the anodic peak at − 0.3 V is related to the hydrogen oxidation during electrochemical measurements [26, 46]. Moreover, for the Bi2S3-EW10 and Bi2S3-EW01 samples, an additional peak appeared at − 0.78 V, which can be ascribed to the hydrogen desorption processes [47]. As the scan rate increases to 100 mV s−1, a shift of both anodic and cathodic redox peaks can be found, which indicates the change in the internal resistance of the electrode and a quasi-reversible nature of the faradaic reaction. As shown in Fig. 5f, the redox current of the Bi3+/Bi0 couple was proved to be in linear correlation of the square root of scan rate. Such characteristics indicate that the electrochemical behavior on the Bi2S3 electrodes was controlled by diffusion process [48]. The slope of the bismuth sulfides synthesized in the mixture of two solvents was larger than from pure water and ethanol, which indicated a faster diffusion process [49]. These findings reveal that the controlled, more developed crystalline structure strongly influences the electrochemical performance of Bi2S3-based electrode. According to Eftekhari and Mohamedi [50], introduction of the battery-like materials with a less crystalline structure allows to distribute redox reactions over wider potential range. Thus, electrode materials with Nernstian features exhibit improved pseudocapacitive performance, which leads to the better electrochemical response at the fast scan rates.
Galvanostatic charge–discharge profiles of the bismuth sulfide samples at a current density of 2 A g−1 and 10 A g−1 are presented in Fig. 6a, b, respectively. All materials exhibit a non-linear charge–discharge curve with a battery-like behavior, which is in accordance with the CV results [51]. At a current density of 2 A g−1, two clearly visible plateaus are observed for the Bi2S3 synthesized with the presence of water, attributed to the faradaic cathodic and anodic peaks around − 0.65 and − 0.4 V, respectively. In case of the Bi2S3 obtained in pure ethanol, the charge–discharge curve present a different behaviour. An additional discharge plateau around − 0.8 V appears, related to the hydrogen desorption process. With the increase of the current density to 10 A g−1, significant changes in the discharge curve occur for the Bi2S3-EW11, as the slope becomes more capacitive in nature [52]. This indicates that the contribution of the faradaic reactions of Bi2S3 to the overall capacity decreases. The Bi2S3-EW12 presents the longest charge–discharge time among all samples, thus confirming that the formed crystalline structure and hexagonal nanoplatelet morphology endure high current regimes and provide higher specific capacity values. At a scan rate of 1 mV s−1, Bi2S3-EW12 achieves a specific capacity of 1022 C g−1, while the bismuth sulfides synthesized at ethanol-to-water ratio of 1:0, 2:1, 1:1, and 0:1 exhibited specific capacities of 810, 902, 794, and 785 C g−1 (Fig. 6c). Additionally, relationship between specific capacity and discharge current density of the Bi2S3 electrodes are presented in Fig. 6d. The best electrochemical performance is observed for the Bi2S3-EW12 electrode, which exhibits the highest capacity values of 748 and 186 C g−1 in the current density range of 0.5–20 A g−1, respectively. The specific capacity of Bi2S3-EW10, Bi2S3-EW21, Bi2S3-EW11, and Bi2S3-EW01 at a current density of 0.5 A g−1 was 681, 693, 551, and 443 C g−1, respectively. Moreover, bismuth sulfide prepared in the solvent mixture of ethanol and water with 1:2 ratio presents the best rate capability among all samples, maintaining 25% of initial capacity at a high current density of 20 A g−1. Specific capacity of the Bi2S3-EW12 excels among other reported bismuth sulfides and their composites synthesized via different methods, due to the stable electrochemical performance in a wide working potential window (Table 2).
For further understanding of the electrochemical properties of the synthesized Bi2S3 nanostructures, EIS measurements were performed and presented in the form of Nyquist plots (Fig. 7). Very low values of bulk resistance RS in the range of 0.1–0.19 Ω for the synthesized bismuth sulfides indicate their good conductive properties. In the low-frequency region, the more inclined plot is towards to − Z″ axis, the lower is the resistance attributed to the diffusion of ions at the electrode/electrolyte interface [26]. The Bi2S3-EW01 prepared in water-only environment presents the highest Warburg resistance among all samples, which suggests that fully crystalline structure is not preferred for an efficient transport of ions. These results are in a good agreement with CV and galvanostatic measurements, and indicate poorer electrochemical performance of the Bi2S3-EW01 compared with Bi2S3 nanostructures prepared from mixed ethanol–water solvents. Moreover, Ramasamy et al. [55] confirmed that nanoplate-like morphology is beneficial for effective insertion and extraction of the electrolyte ions.
Conclusions
In summary, the solvent system is a crucial parameter in the synthesis of the Bi2S3 nanostructures. Adjusting the volume ratio of ethanol--water mixture allows to control not only the bismuth sulfide morphology, but also the degree of a crystallinity of the orthorhombic structure. With the increasing amount of organic solvent, Bi2S3 synthesized via facile chemical precipitation method becomes more amorphous. Electrochemical performance of the electrodes based on the bismuth sulfide nanostructures is related to their crystalline structure and morphology. The highest specific capacity of 748 C g−1 at a current density of 0.5 A g−1 was achieved by Bi2S3 synthesized in a mixture of ethanol and water with a volume ratio of 1:2, respectively. Additionally, Bi2S3-EW12 exhibited the best rate capability under increasing current density to 20 A g−1 among all the investigated samples. Such electrochemical performance may be related to the appropriate structure with low crystallinity degree and to the hexagonal nanoplatelet morphology, which enhances battery-like behaviour at low and high currents. The following work encourages the design of new hybrid materials based on bismuth sulfides for future energy storage devices such as supercapatteries and hybrid capacitors.
References
Jang, Y., Jo, J., Choi, Y.-M., Kim, I., Lee, S.-H., Kim, D., Yoon, S.M.: Activated carbon nanocomposite electrodes for high performance supercapacitors. Electrochim. Acta 102, 240–245 (2013)
Śliwak, A., Grzyb, B., Ćwikła, J., Gryglewicz, G.: Influence of wet oxidation of herringbone carbon nanofibers on the pseudocapacitance effect. Carbon 64, 324–333 (2013)
Pandolfo, A.G., Hollenkamp, A.F.: Carbon properties and their role in supercapacitors. J. Power Sources 157, 11–27 (2006)
Moyseowicz, A., Śliwak, A., Miniach, E., Gryglewicz, G.: Influence of structural and textural parameters of carbon nanofibers on their capacitive behavior. J. Mater. Sci. 51, 3431–3439 (2016)
Śliwak, A., Grzyb, B., Diez, N., Gryglewicz, G.: Nitrogen-doped reduced graphene oxide as electrode material for high rate supercapacitors. Appl. Surf. Sci. 399, 265–271 (2017)
Moyseowicz, A., Gryglewicz, G.: Hydrothermal-assisted synthesis of a porous polyaniline/reduced graphene oxide composite as a high-performance electrode material for supercapacitors. Compos. B Eng. 159, 4–12 (2019)
Gao, F., Qu, J., Zhao, Z., Wang, Z., Qiu, J.: Nitrogen-doped activated carbon derived from prawn shells for high-performance supercapacitors. Electrochim. Acta 190, 1134–1141 (2016)
Gryglewicz, G., Śliwak, A., Beguin, F.: Carbon nanofibers grafted on activated carbon as an electrode in high-power supercapacitors. Chemsuschem 6, 1516–1522 (2013)
Zhang, X., Yu, P., Zhang, H., Zhang, D., Sun, X., Ma, Y.: Rapid hydrothermal synthesis of hierarchical nanostructures assembled from ultrathin birnessite-type MnO2 nanosheets for supercapacitor applications. Electrochim. Acta 89, 523–529 (2013)
Babu, I.M., William, J.J., Muralidharan, G.: Hierarchical β-Co(OH)2/CoO nanosheets: an additive-free synthesis approach for supercapattery applications. Ionics 25, 2437–2444 (2019)
Śliwak, A., Gryglewicz, G.: High-voltage asymmetric supercapacitors based on carbon and manganese oxide/oxidized carbon nanofiber composite electrodes. Energy Technol. 2, 819–824 (2014)
Miniach, E., Śliwak, A., Moyseowicz, A., Fernández-Garcia, L., González, Z., Granda, M., Menendez, R., Gryglewicz, G.: MnO2/thermally reduced graphene oxide composites for high-voltage asymmetric supercapacitors. Electrochim. Acta 240, 53–62 (2017)
Iqbal, J., Numan, A., Rafique, S., Jafer, R., Mohamad, S., Ramesh, K., Ramesh, S.: High performance supercapattery incorporating ternary nanocomposite of multiwalled carbon nanotubes decorates with Co3O4 nanograins and silver nanoparticles as electrode material. Electrochim. Acta 278, 72–81 (2018)
Yu, L., Chen, G.Z.: Redox electrode materials for supercapatteries. J. Power Sources 326, 604–612 (2016)
Zhang, Z., Huang, Z., Ren, L., Shen, Y., Qi, X., Zhong, J.: One-pot synthesis of hierarchically nanostructured Ni3S2 dendrites as active materials for supercapacitors. Electrochim. Acta 149, 316–323 (2014)
Wei, W., Mi, L., Gao, Y., Zheng, Z., Chen, W., Guan, X.: Partial ion-exchange of nickel-sulfide-derived electrodes for high performance supercapacitors. Chem. Mater. 26, 3418–3426 (2014)
Huang, K.-J., Zhang, J.Z., Shi, G.W., Liu, Y.-M.: One-step hydrothermal synthesis of two-dimensional cobalt sulfide for high-performance supercapacitors. Mater. Lett. 131, 45–48 (2014)
Chen, W., Xia, Z., Alshareef, H.N.: One-step electrodeposited nickel cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 8, 9531–9541 (2014)
Tie, J., Han, J., Diao, G., Liu, J., Xie, Z., Cheng, G., Sun, M., Yu, L.: Controllable synthesis of hierarchical nickel cobalt sulfide with enhanced electrochemical activity. Appl. Surf. Sci. 435, 187–194 (2018)
Yang, H., Xie, J., Li, C.M.: Bi2S3 nanorods modified with Co(OH)2 ultrathin nanosheets to significantly improve its pseudocapacitance for high specific capacitance. RSC Adv. 4, 48666–48670 (2014)
Durga, I.K., Rao, S.S., Reddy, A.E., Gopi, C.V.V.M., Kim, H.-J.: Achieving copper sulfide leaf like nanostructure electrode for high performance supercapacitor and quantum-dot sensitized solar cells. Appl. Surf. Sci. 435, 666–675 (2018)
Wang, S.F., Wang, W., Fong, W.K., Yu, Y., Surya, C.: Tin compensation for the SnS based optoelectronic devices. Nat. Sci. Rep. 7, 39704 (2017)
Yang, X., Tian, S., Li, R., Wang, W., Zhou, S.: Use of single-crystalline Bi2S3 nanowires as room temperature ethanol sensor synthesized by hydrothermal approach. Sens. Actuators B Chem. 241, 210–216 (2017)
Ma, L., Zhao, Q., Zhang, Q., Ding, M., Huang, J., Liu, X., Liu, Y., Wu, X., Xu, X.: Controlled assembly of Bi2S3 architectures as Schottky diode, supercapacitor electrodes and highly efficient photocataysts. RSC Adv. 4, 41636–41641 (2014)
Arumugam, J., Raj, A.D., Irudayaraj, A.A.: Reaction time dependent investigation on the Bi2S3 nanoparticles: photocatalytic application. Mater. Today Proc. 5, 16094–16099 (2018)
Moyseowicz, A.: Scalable one-pot synthesis of bismuth sulfide nanorods as an electrode active material for energy storage applications. J. Solid State Electrochem. 23, 1191–1199 (2019)
Jana, A., Bhattacharya, C., Sinha, S., Datta, J.: Study of the optimal condition for electroplating of Bi2S3 thin films and their photoelectrochemical characteristics. J. Solid State Electrochem. 13, 1339–1350 (2009)
Liu, K.L., Chen, F., Liu, Y., Li, D., Shi, W.D.: Synthesis of hierarchical Bi2S3 nanoflowers via a topotatic transformation from hierarchical Bi2WO6 nanoflowers and their supercapacitor performance. CrystEngComm 19, 570–575 (2017)
Liang, K., Wang, C., Xu, X., Leng, J., Ma, H.: Capacitive and photocatalytic performance of Bi2S3 nanostructures synthesized by solvothermal method. Phys. Lett. A 381, 652–657 (2017)
Vadivel, S., Naveen, A.N., Kamalakannan, V.P., Cao, P., Balasubramanian, N.: Facile large scale synthesis of Bi2S3 nanorods-graphene composite for photocatalytic photoelectrochemical and supercapacitor application. Appl. Surf. Sci. 351, 635–645 (2015)
Chai, W., Yang, F., Yin, W., You, S., Wang, K., Ye, W., Rui, Y., Tang, B.: Bi2S3/C nanorods as efficient anode materials for lithium-ion batteries. Dalton Trans. 48, 1906–1914 (2019)
Yue, H., Chen, S., Li, P., Zhu, C., Yang, X., Li, T., Gao, Y.: Lemongrass-like Bi2S3 as a high-performance anode material for lithium-ion batteries. Ionics 25, 3587–3592 (2019)
Kumari, P., Awasthi, K., Agarwal, S., Ichikawa, T., Kumar, M., Jain, A.: Flower-like Bi2S3 nanostructures as highly efficient anodes for all-solid-state lithium-ion batteries. RSC Adv. 9, 29549–29555 (2019)
Lee, H.W., Shginde, N.M., Shinde, P.V., Yun, J.M., Song, P.K., Kim, K.H.: High energy and power density of self-grown CuS@Cu2O core-shell supercapattery positrode. J. Solid State Electrochem. 23, 2609–2617 (2019)
Peng, X.S., Meng, G.W., Zhang, J., Zhao, L.X., Wang, X.F., Wang, Y.W., Zhang, L.D.: Electrochemical fabrication of ordered Bi2S3 nanowire arrays. J. Phys. D Appl. Phys. 34, 3224–3228 (2001)
Sheng, Q., Shen, Y., Wu, Q., Zheng, J.: Direct electrochemistry and electrocatalysis of cytochrome c based on dandelion-like Bi2S3 nanoflowers. J. Solid State Electrochem. 20, 3315–3322 (2016)
Liao, X.H., Wang, H., Zhu, J.J., Chen, H.-Y.: Preparation of Bi2S3 nanorods by microwave irradiation. Mater. Res. Bull. 36, 2339–2346 (2001)
Chen, Y., Kou, H., Jiang, J., Su, Y.: Morphologies of nanostructured bismuth sulfide prepared by different synthesis routes. Mater. Chem. Phys. 82, 1–4 (2003)
Tian, L., Tan, H.Y., Vittal, J.J.: Morphology-controlled synthesis of Bi2S3 nanomaterials via single- and multiple-source approaches. Cryst. Growth Des. 8, 734–738 (2008)
Panigrahi, P.K., Pathak, A.: The growth of bismuth sulfide nanorods from spherical-shaped amorphous precursor particles under hydrothermal condition. J. Nanoparticles 367812 (2013)
Peng, H., Ma, G., Mu, J., Sun, K., Lei, Z.: Controllable synthesis of CuS with hierarchical structures via a surfactant-free method for high-performance supercapacitors. Mater. Lett. 122, 25–28 (2014)
Park, J.Y., Jung, H.C., Raju, G.S.R., Moon, B.K., Jeong, J.H., Choi, H.Y., Kim, J.H.: Facile solvothermal synthesis and polarity based tunable morphologies of ZnO nanocrystals. Ceram. Int. 39, 6599–6606 (2013)
Li, W.H.: Synthesis and characterization of bismuth sulfide nanowires through microwave solvothermal technique. Mater. Lett. 62, 243–245 (2008)
Yang, X.Y., Wang, X., Zhang, Z.: Facile solvothermal synthesis of single-crystalline Bi2S3 nanorods on a large scale. Mater. Chem. Phys. 95, 154–157 (2006)
Lou, W., Chen, M., Wang, X., Liu, W.: Novel single-source precursors approach to prepare highly uniform Bi2S3 and Sb2S3 nanorods via a solvothermal treatment. Chem. Mater. 19, 872–878 (2007)
Zhang, B., Ye, X., Hou, W., Zhao, Y., Xie, Y.: Biomolecule assisted synthesis and electrochemical hydrogen storage of Bi2S3 flowerlike patterns with well-aligned nanorods. J. Phys. Chem. B 110, 8978–8985 (2006)
Duca, M., Guerrini, E., Colombo, A., Trasatti, S.: Activation of nickel for hydrogen evolution by spontaneous deposition of iridium. Electrocatalysis 4, 338–345 (2013)
Waite, T.J., Kraiya, C., Trouwborst, R.E., Ma, S., Luther, G.W.: An investigation into the suitability of bismuth as an alternative to gold-amalgam as a working electrode for the in situ determination of chemical redox species in the natural environment. Electroanalysis 18, 1167–1172 (2006)
Zhou, H., Xi, J., Li, Z., Zhang, Z., Yu, L., Liu, L., Qiu, X., Chen, L.: CeO2 decorated graphite felt as a high-performance electrode for vanadium redox flow batteries. RSC Adv. 4, 61912–61918 (2014)
Eftekhari, A., Mohamedi, M.: Tailoring pseudocapacitive materials from a mechanistic perspective. Mater. Today Energy 6, 211–229 (2017)
Dubal, D.P., Ayyad, O., Ruiz, V., Gómez-Romero, P.: Hybrid energy storage: the merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 44, 1777–1790 (2015)
Zhao, B., Chen, D., Xiong, X., Song, B., Hu, R., Zhang, Q., Rainwater, B.H., Waller, G.H., Zhen, D., Ding, Y., Chen, Y., Qu, C., Dang, D., Wong, C.P., Liu, M.: A high-energy, long cycle-life hybrid supercapacitor based on graphene composite electrodes. Energy Storage Mater. 7, 32–39 (2016)
Nie, G., Lu, X., Lei, J., Yang, L., Wang, C.: Facile and controlled synthesis of bismuth sulfide nanorods-reduced graphene oxide composites with enhanced supercapacitor performance. Electrochim. Acta 154, 24–30 (2015)
Lu, H., Guo, Q., Zan, F., Xia, H.: Bi2S3 nanoparticles anchored on graphene nanosheets with superior electrochemical performance for supercapacitors. Mater. Res. Bull. 96, 471–477 (2017)
Ramasamy, K., Gupta, R.K., Sims, H., Palchoudhury, S., Ivanov, S., Gupta, A.: Layered ternary sulfide CuSbS2 nanoplates for flexible solid-state supercapacitors. J. Mater. Chem. A 3, 13263–13274 (2015)
Acknowledgements
The research leading to above results has received funding from the National Science Centre (Poland) under the Grant agreement 2016/21/D/ST5/01642.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moyseowicz, A., Moyseowicz, A. Tailoring the morphology, crystalline structure, and electrochemical properties of nanostructured Bi2S3 using various solvent mixtures. Mater Renew Sustain Energy 9, 11 (2020). https://doi.org/10.1007/s40243-020-00171-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-020-00171-9