Abstract
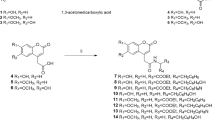
Sixteen novel and four known(4a, 4d, 4e, 4h) amine derivatives of furocoumarin were synthesized, then submitted to evaluation as stimulators of melanogenesis and tyrosinase in B16 murine cells. Among them, five compounds(4g, 4j―4m) showed potent activating effect on both melanogenesis and tyrosinase in vitro compared with positive control(8-MOP), the most widely used drugs for vitiligo in clinic. Noticeably, compounds 4h and 4j, which contain morpholine and piperazine, were recognized as the most effective stimulator of melanogenesis and tyrosianse in B16 cells separately. These derivatives may serve as lead compounds for further drug discovery for the treatment of vitiligo.
Similar content being viewed by others
References
Ezzedine K., Sheth V., Rodrigues M., Eleftheriadou V., Harris J. E., Hamzavi I. H., Pandya A. G., J. Am. Acad. Dermatol., 2015, 73(5), 883
Ezzedine K., Lim H. W., Suzuki T., Katayama I., Hamzavi I., Lan C. C., Goh B. K., Anbar T., Silva de Castro C., Lee A.Y., Parsad D., van Geel N., Poole I. C., Oiso N., Benzekri L., Spritz R., Gauthier Y., Hann S. K., Picardo M., Taieb A., Pigm. Cell Melanoma Res. 2012, 25(3), E1
Harris J. E., J. Invest. Dermatol., 2015, 135(12), 2921
Alikhan A., Felsten L., Daly M., Petronic-Rosic V., J. Am. Acad. Dermatol., 2011, 65(3), 473
Laddha N. C., Dwivedi M., Mansuri M. S., Gani A. R., Ansarullah M., Ramachandran A.V., Dalai S., Begum R., Exp. Dermatol. 2013, 22(4), 245
Spritz R. A., J. Invest. Dermatol., 2011, 131(3), e18
Sandoval-Cruz M., García-Carrasco, M. S ánchez-Porras R., Mendo-za-Pinto C., Jiménez-Hernández M., Munguía-Realpozo P., Ruiz-Argūelles A., Autoimmun Rev. 2011, 10(12), 762
Garcia-Molina M. M., Muñoz-Muñoz J. L., Garcia-Molina F., García-Ruiz P. A., Garcia-Canovas F., J. Agric. Food Chem., 2012, 60(25), 6447
Ismaya W. T., Rozeboom H. J., Weijn A., Mes J. J., Fusetti F., Wichers H. J., Dijkstra B. W., Biochem. 2011, 50(24), 5477
Wu C. Y., Sun Z. P., Ye Y. Y., Han X. H., Song X.Y., Liu S., Fitotera-pia 2013, 91, 205
Fitzpatrick T. B., Pathak, M. A., J. Invest. Dermatol. 1959, 32(2), 229
Yao L., Li Q., Shang J., J. Xinjiang Med. Univ., 2010, 33(10), 1191
Zhou J., Shang J., Ping F. F., Zhao G. R., J. Ethnopharmacol., 2012, 143(2), 639
Jois H. S., Manjunath B. L., Venkata R. S., J. Indian Chem. Soc., 1933, 10, 45
Späth E., Ber. Deut. Chem. Ges. 1937, 70, 83
El Mofty A. M., Vitiligo and Psoralens, Pergamon Press, Oxford, 1968, 1147
Fitzpatrick T. B., Parrish J. A., Pathak M. A., Phototherapy of Vitili-go(Idiopathic leukoderma) in Sunlight and Man, Tokyo University Press, Tokyo, 1974, 783
Whitton M. E., Ashcroft D. M., González U. J., Am. Acad. Dermatol. 2008, 59(4), 713
Felsten L. M., Alikhan A., Petronic-Rosic V., J. Am. Acad. Dermatol., 2011, 65(3), 493
Tippisetty S., Goudi D., Mohammed A. W., Jahan P., Toxicol. In Vitro, 2013, 27(1), 38
Kerns E. H., Di L., Drug Discov. Today 2003, 8(7), 316
Dreassi E., Zizzari A. T., Mori M., Filippi I., Belfiore A., Naldini A., Carraro F., Santucci A., Schenone S., Botta M., Eur. J. Med. Chem., 2010, 45(12), 5958
Niu C., Pang G. X., Li G., Dou J., Nie L. F., Himit H., Kabas M., Aisa H. A., Bioorg. Med. Chem. 2016, 24(22), 5960
Nakamura T., Wada H., Kurebayashi H., McInally T., Bonnert R., Isobe Y., Bioorg. Med. Chem. Lett. 2013, 23(3), 669
Sensi M., Catani M., Castellano G., Nicolini G., Alciato F., Tragni G., De Santis G., Bersani I., Avanzi G., Tomassetti A., Canevari S., Ani-chini S., J. Invest. Dermatol. 2011, 131(12), 2448
Roh E., Yun C. Y., Yun J. Y., Park D., Doo K. N., Yeon H. B., Jung S. H., Park S. K., Kim Y. B., Han S. B., Kim Y., J. Invest. Dermatol., 2013, 133(4), 1072
Binda C., Wang J., Pisani L., Caccia C., Carotti A., Salvati P., Edmondson D. E., Mattevi A., J. Med. Chem., 2007, 50(23), 5848
Chilin A., Manzini P., Caffieri S., Rodighiero P., Guiotto A., J. Heterocycl. Chem., 2001, 38(2), 431
Patel J. M., Soman S. S., J. Heterocycl. Chem., 2010, 47(2), 379
Chattopadhyay K., Fenster E., Grenning A. J. J. Tunge J. A., Beilstein J. Org. Chem., 2012, 8(133), 1200
Kim H. J., Kim J. S., Woo J. T., Lee I. S., Cha B. Y., Acta Biochim. Biophys. Sin. 2015, 47(7), 548
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Natural Science Foundation of Xinjiang, China(No. 2017D01A76).
Electronic supplementary material
40242_2018_7338_MOESM1_ESM.pdf
Design, Synthesis and Biological Activity of Novel Furocoumarin Derivatives as Activator of Melanogenesis and Tyrosinase in B16 Cells
Rights and permissions
About this article
Cite this article
Niu, C., Zang, D. & Aisa, H.A. Design, Synthesis and Biological Activity of Novel Furocoumarin Derivatives as Stimulators of Melanogenesis and Tyrosinase in B16 Cells. Chem. Res. Chin. Univ. 34, 408–414 (2018). https://doi.org/10.1007/s40242-018-7338-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-018-7338-4