Abstract
Phototherapy with psoralens as photosensitizers is the commonly used method for the treatment of vitiligo. Considering the structure similarity between psoralens and thieno[2,3-d]pyrimidinones, a series of novel sulfonamide derivatives containing tricyclic thieno[2,3-d]pyrimidinone were synthesized and evaluated for melanin synthesis in murine B16 cells. All new compounds were characterized by 1H NMR, 13C NMR, IR and HRMS (ESI). Among them, 6 compounds demonstrated excellent activity than positive control (8-methoxylpsoralen, 8-MOP) with more than 1.5-fold potency. Compound 11w with dichloro substitution at meta-positions in the benzenesulfonyl moiety was the most potent one (658.3 ± 8.7%), exhibiting 5.0-fold stronger activity than 8-MOP (130.9 ± 8.6%). The difluoro analog compound 11o increased melanin synthesis in murine B16 cells with a 4.35-fold potency as compared to 8-MOP. These compounds may serve as lead compounds for further drug discoveries for the treatment of vitiligo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitiligo, the most frequent cause of skin depigmentation, is a chronic disorder clinically characterized by the development of white macules due to the loss of cutaneous melanocytes [1]. Although vitiligo is not as fatal as other diseases like heart and Alzheimer’s disease, it could be of severe cosmetic disfiguration and the patients usually also suffer from psychological devastation and social stigmatization. Overall, around 0.06–2.28% of the world’s population is affected by vitiligo [2], but its etiology remains elusive. It is believed that auto-immune or auto-inflammation is the leading cause, based on the clinical association of vitiligo with other auto-immune disorders [3]. Several other hypotheses have also been elaborated, but not universally accepted, including genetic mutation, skin trauma, neural theory, reactive oxygen species, and melanocytorrhagy hypothesis. Interacted factors are suggested to trigger and contribute to the development of vitiligo lesions.
Clinically, several drugs for treating vitiligo have been applied to stimulate melanocyte proliferation and/or to improve melanin biosynthesis within melanocytes, such as topical corticosteroids, calcineurin inhibitors, vitamin D3 analogs, and psoralens (with UV raidation) [4]. These treatments, although they cannot cure vitiligo, might adjust melanogenesis, a multistage process stimulated by various effectors including ultraviolet light, and then help the repigmentation of vitiligo lesions [5].
Psoralens (also named furocoumarins) are a class of heterocyclic compounds with a known phototherapeutic activity which have been isolated from several natural plants such as Ammi majus and Psoralea corylifolia [6]. The most effective psoralen is 8-methoxylpsoralen (8-MOP, 1, Fig. 1). It is argued that 8-MOP-mediated phototherapy might stimulate melanocyte proliferation [7] and enhance enzymatic activity [8], although precise mechanisms still remain unknown. Psoralens, although frequently used [9], suffer from low water solubility and carcinogenic hazards in some cases [10]. Therefore, the development of new photosensitizers is of general interest for vitiligo treatment.
Thieno[2,3-d]pyrimidin-4(3H)-ones have been widely applied in medicinal chemistry owing to their diverse biological functions [11, 12]. For example, eggmaone (2, Fig. 2) has been reported to inhibit Hedgehog signaling pathway for the treatment of cancers [13]. Endo et al. [14] developed a series of 2-(isopropylamino)thieno[3,2-d]pyrimidin-4(3H)-one derivatives as selective phosphodiesterase 7 inhibitors, with compound 3 (Fig. 2) as the most potent one for treating inflammation disease. ICL-SIRT078 (4, Fig. 2), was found to be a competitive sirituin 2 inhibitor with a K i value of 0.62 ± 0.15 μM, and is also highly neuroprotective and might become a candidate for the treatment of Parkinson’s disease [15]. Compound 5 containing a 1,2,4-triazole moiety shows pronounced cytotoxicity on human colorectal cancer cell line HT-29 and cervical cancer cell HeLa [16], but there are not reports on the synthesis of derivatives of thieno[2,3-d]pyrimidin-4(3H)-ones as photosensitizers for the treatment of vitiligo.
The development of new therapeutic agents for vitiligo is one of the long-term research topics of our group. Recently, we have synthesized dozens of isoxazole chalcones [17] and psoralens derivatives [18, 19] possessing stimulation effects on tyrosinase and melanin synthesis in murine B16 cells. Considering the structural similarity between thieno[2,3-d]pyrimidinone and psoralens (Fig. 1), we decided to synthesize some new sulfonamides containing tricyclic thieno[2,3-d]pyrimidinone moieties in order to study their effects on melanogenesis in murine B16 cells.
Experimental
Reagents and solvents were purchased from Sigma-Aldrich, and used without further purification. Thin-layer chromatography (TLC) was carried out on glass plates coated with silica gel (G60F-254; Qingdao Haiyang Chemical) and visualized by UV light (254 nm). The products were purified by column chromatography over silica gel (200–300 mesh; Qingdao Haiyang Chemical). Melting points were determined on a Buchi B-540 apparatus and uncorrected. All the NMR spectra were recorded with a Varian 400 MHz NMR spectrometer in CDCl3, using TMS as an internal standard. High-resolution mass spectra (HRMS) were recorded on a AB SCIEX QSTAR Elite quadrupole time-of-flight mass spectrometer. The IR data were recorded on a Thermo Fisher Scientific Nilolet 6700 FT-IR infrared spectrometer (KBr). The detailed synthetic procedure and spectral data of intermediates 6–10 were recorded in the electronic supplementary material associated with this article.
General procedure of preparation of (11a–11x)
To a mixture of 3-amino-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]thieno[2,3-d]pyrimidin-4-one (1.0 mmol) (10) and pyridine (1.2 mmol) in CH2Cl2 (2 mL), benzenesulfonyl chloride (1.1 mmol) in CH2Cl2 (10 mL) was added dropwise. The resulting mixture was stirred at room temperature for 12 h. The mixture was concentrated under reduced pressure, and the residue was diluted with water (30 mL). The aqueous mixture was neutralized by the addition of aqueous 10% HCl solution and extracted with CH2Cl2 (2 × 30 mL). The organic phase was washed with aqueous saturated NH4Cl solution and brine. The organic layer was separated and dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to give the crude product, which was purified by silica gel chromatography to produce the pure corresponding compounds. The full structual elucidation of compounds 11a–11x can be found in the electronic supplementary material associated with this article.
Biological activity
Cell culture
Murine B16 melanoma cell lines (B16F10) were obtained from Chinese Academy of Sciences, China. The B16F10 cells were grown in DMEM medium (Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 U/mL penicillin and 100 mg/mL streptomycin (Gibco) in a humidified atmosphere with 5% CO2 at 37 °C.
Melanin contents assay
Exponentially growing cells were seeded into 6-well plates at a concentration of 5 × 105 cells per well. After 24 h incubation at 37 °C, the culture medium was removed and replaced with fresh medium containing the candidate compounds of 50 μM concentration. The cells were incubated for another 48 h, washed with ice-cold PBS, followed by lysis with RIPA buffer for 40 min on ice, and the lysates were centrifuged at 10,000 g for 20 min. Supernatants containing protein were subject to a protein assay and the pellets with intracellular melanin were solubilized in 200 μL of 1 M NaOH for 2 h at 60 °C. Melanin amounts were determined spectrophotometrically at 405 nm by a multi-plate reader. The themelanin amount was calculated by normalizing the total melanin values with protein content (abs melanin/L g protein).
Results and discussion
Synthesis
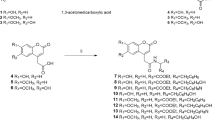
Chemical preparation of the target compounds is shown in Scheme 1. Diethyl oxalate was treated with ethylmagnesium bromide to give ethyl 2-oxobutanoate (6). Condensation of ethyl 2-oxobutanoate (6) with ethyl cyanoacetate and sufur in ethanol in the presence of trimethylamine yielded 2-aminothiophene (7) [20, 21], which was further condensed with δ-valerolactam to afford the intermediate ethyl thieno[2,3-d]pyrimidine-3-carboxylate 8 [22]. Hydrolysis of ester 8 was completed in water and methanol with excessive amounts of lithium hydroxide. Application of Curtius rearrangement to the acid 9 using diphenylphosphorylazide in tert-butanol and following acdified hydrolysis resulted in the amine 10.
The rearrangement of intermediates 9–10 was the crucial step for the preparation of compounds 11a–11x. A number of rearrangement conditions, such as Lossen rearrangement, Schmidt rearrangement and Curtius rearrangement were tried to improve the yield of 10 (Table 1).
Lossen rearrangement of the corresponding acids 9 at the presence of hydroxylamine hydrochloride in polyphosphoric acid at 150 °C gave compounds 10 in moderate yield (58%, Table 1, Entry 1). Schmidt rearrangement method (sodium azide and sulfuric acid) gave converted acids 9 into amine 10 in 70% yield at room temperature (Table 1, Entry 2), slightly better than Lossen rearrangement. The best yield (91%) was achieved with the application of Curtius rearrangement. In this case, compound 9 was smoothly transformed to 10 with diphenylphosphorylazide in tert-butanol under nitrogen atmosphere followed by deprotection with HCl in ethanol. Finally, the target sulfonamides 11a–11x were synthesized by condensation of amine 10 with appropriate aromatic sulfonyl chlorides in dichloromethane with good to excellent yields (72–92%).
All new compounds were characterized by 1H NMR, 13C NMR, IR and HRMS (ESI). The structures of compounds 11o and 11w were further confirmed by X-ray diffraction analysis as shown in Fig. 3 (11o: CCDC No.: 1528441; 11w: CCDC No. 1528442) [23]. These two single crystals were obtained by slow evaporation of ethanol at room temperature.
Melanin synthesis evaluation
All the thieno[2,3-d]pyrimidine derivatives 11a–11x were screened for their activity on melanin synthesis in murine B16 cells, using a previously published method [24].
According to the results shown in Table 2, in general, the substitution pattern of the benzene ring of the sulfonamide moiety dramatically influenced the activity on melanin synthesis. The activities greatly improved when the compounds were substituted by –NO2, –CF3, –OCF3, –F, and –Cl groups at the benzene ring of the sulfonamide moiety, which suggested that the electron-withdrawing group might be favorable to enhance the activity.
Compounds 11d (448.2 ± 0.8%), 11o (570.5 ± 10.1%) and 11w (658.3 ± 8.7%) of the derivatives with a six-membered ring displayed the best activities. Among the mono- and difluoro-substituted derivatives (11c–11e, 11o–11s), the most important factor in their efficacy was the location of the group. The shift of the fluorine atom from the meta (11d, 448.2 ± 0.8%) into the ortho and para positions led to 11c (107.1 ± 7.4%) and 11e (105.2 ± 7.2%), respectively, which had a 4.2-fold lower activity. The introduction of a second fluorine atom to the meta position of the benzene ring strongly increased the activity, exemplified by 11d (448.2 ± 0.8%), 11e (105.2 ± 7.2%) and 11c (107.1 ± 7.4%), as compared with 11o (570.5 ± 10.1%), 11p (227.8 ± 6.2%) and 11r (171.5 ± 5.2%). 3.5-Dichloro-substituted 11w (658.3 ± 8.7%) exhibited the best activity compared with all the others, being even more potent than 3,5-difluoro 11o (570.5 ± 10.1%). From the above results, it can be concluded that the presence of halogen (–F or –Cl) on meta-position of benzene is crucial for maintaining the good activity.
Conclusion
In summary, a novel series of sulfonamides containing tricyclic thieno[2,3-d]pyrimidinone were synthesized and evaluated for their ability of stimulation on melanin synthesis in murine B16 cells. Preliminary SARs indicated that compounds with –F and –Cl groups at the meta-position of the benzensulfonyl fragment demonstrated better activity than the other prepared compounds. In addition, the most important factor in their efficacy was the location of the group among these mono- and di-halogenated compounds. It was of note that the activity of 11o (570.5 ± 10.1%) and 11w (658.3 ± 8.7%) were nearly 4.4- and 5.0-fold stronger than 8-MOP (130.9 ± 8.6%) on melanin synthesis in murine B16 cells. The investigated results suggest that several 5-sulfonamide-containing tricyclic thieno[2,3-d]pyrimidinones revealed promising activity on melanin synthesis in murine B16 cells and may serve as lead compounds for further drug discoveries and the treatment of vitiligo.
Further preparation and evaluation of other tricyclic thieno[2,3-d]pyrimidinone derivatives is ongoing and will be reported in due course.
References
K. Ezzedine, V. Eleftheriadou, M. Whitton, N. van Geel, Lancet 386, 74 (2015)
C. Krüger, K.U. Schallreuter, Int. J. Dermatol. 51, 1206 (2012)
G.F. Mohammed, A.H. Gomaa, M.S. Al-Dhubaibi, World J. Clin. Cases 3, 221 (2015)
L.M. Felsten, A. Alikhan, V. Petronic-Rosic, J. Am. Acad. Dermatol. 65, 493 (2011)
I.F. Videira, D.F. Moura, S. Magina, An. Bras. Dermatol. 88, 76 (2013)
M.A. Pathak, T.B. Fitzpatrick, J. Photochem. Photobiol. B 14, 3 (1992)
M.B. Abdel-Naser, S.K. Hann, J.C. Bystryn, Arch. Dermatol. 133, 1530 (1997)
C.S. Wu, C.C. Lan, L.F. Wang, G.S. Chen, C.S. Wu, H.S. Yu, Br. J. Dermatol. 156, 122 (2007)
P. Wolf, Br. J. Dermatol. 174, 11 (2016)
E. Archier, S. Devaux, E. Castela, A. Gallini, F. Aubin, M. Le Maitre, S. Aractingi, H. Bachelez, B. Cribier, P. Joly, D. Jullien, L. Misery, C. Paul, J.P. Ortonne, M.A. Richard, J. Eur. Acad. Dermatol. Venereol. 26(Suppl 3), 22 (2012)
K. Bozorov, J.Y. Zhao, B. Elmuradov, A. Pataer, H.A. Aisa, Eur. J. Med. Chem. 102, 552 (2015)
A.A. Aly, E.A. Ishak, M. Ramadan, M.O. Germoush, T.I. El-Emary, N.S. Al-Muaikel, J. Heterocycl. Chem. 50, 451 (2013)
J.E. Hempel, A.G. Cadar, C.C. Hong, Bioorg. Med. Chem. Lett. 26, 1947 (2016)
Y. Endo, K. Kawai, T. Asano, S. Amano, Y. Asanuma, K. Sawada, Y. Onodera, N. Ueo, N. Takahashi, Y. Sonoda, N. Kamei, T. Irie, Bioorg. Med. Chem. Lett. 25, 1910 (2015)
P. Di Fruscia, E. Zacharioudakis, C. Liu, S. Moniot, S. Laohasinnarong, M. Khongkow, I.F. Harrison, K. Koltsida, C.R. Reynolds, K. Schmidtkunz, M. Jung, K.L. Chapman, C. Steegborn, D.T. Dexter, M.J. Sternberg, E.W. Lam, M.J. Fuchter, ChemMedChem 10, 69 (2015)
A. Mavrova, D. Wesselinova, J.A. Tsenov, L.A. Lubenov, Eur. J. Med. Chem. 86, 676 (2014)
C. Niu, G. Li, A. Tuerxuntayi, H.A. Aisa, Chin. J. Chem. 33, 486 (2015)
C. Niu, L. Yin, L.F. Nie, J. Dou, J.Y. Zhao, G. Li, H.A. Aisa, Bioorg. Med. Chem. 24, 5440 (2016)
C. Niu, G.X. Pang, G. Li, J. Dou, L.F. Nie, H. Himit, M. Kabas, H.A. Aisa, Bioorg. Med. Chem. 24, 5960 (2016)
F. Moeinpour, N. Dorostkar, M. Vafaei, Synth. Commun. 42, 2367 (2012)
F. Moeinpour, R. Omidinia, N. Dorostkar-Ahmadi, B. Khoshdeli, Bull. Korean Chem. Soc. 32, 2091 (2011)
A. Lilienkampf, S. Karkola, S. Alho-Richmond, P. Koskimies, N. Johansson, K. Huhtinen, K. Vihko, K. Wähälä, J. Med. Chem. 52, 6660 (2009)
CCDC 1528441 (18o) and 1528442 (18w) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
H.J. Kim, J.S. Kim, J.T. Woo, I.S. Lee, B.Y. Cha, Acta Biochim. Biophys. Sin. 47, 548 (2015)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 21550110495) and the Chinese Academy of Sciences President’s International Fellowship Initiative (Grant No. 2016 PT014).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nie, L.F., Bozorov, K., Niu, C. et al. Synthesis and biological evaluation of novel sulfonamide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones on melanin synthesis in murine B16 cells. Res Chem Intermed 43, 6835–6843 (2017). https://doi.org/10.1007/s11164-017-3023-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3023-3