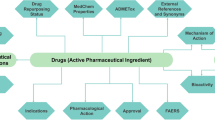
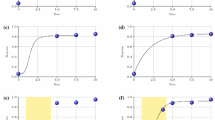
Abstract
Toxicity related failures in drug discovery and clinical development have motivated scientists and regulators to develop a wide range of in-vitro, in-silico tools coupled with data science methods. Older drug discovery rules are being constantly modified to churn out any hidden predictive value. Nonetheless, the dose–response concepts remain central to all these methods. Over the last 2 decades medicinal chemists, and pharmacologists have observed that different physicochemical, and pharmacological properties capture trends in toxic responses. We propose that these observations should be viewed in a comprehensive property-response framework where dose is only a factor that modifies the inherent toxicity potential. We then introduce the recently proposed “Drug Toxicity Index (DTI)” and briefly summarize its applications. A webserver is available to calculate DTI values (https://all-tool-kit.github.io/Web-Tool.html).
Similar content being viewed by others
Notes
As the human organ and body on-chip systems mature, one can expect age-old ethical questions on what constitutes a living organism can be expected to be raised with these technologies. Thus along with biosafety, ethical aspects should be considered early-on in the development and investment in these technologies alongside, dialogues with regulators, academia, global interest groups, and general public at large.
References
Advancing Alternative Methods at FDA | FDA (2021) https://www.fda.gov/science-research/about-science-research-fda/advancing-alternative-methods-fda. Accessed 13 Jan 2021
Aleo MD, Shah F, Allen S, Barton HA, Costales C, Lazzaro S, Leung L, Nilson A, Obach RS, Rodrigues AD et al (2020) Moving beyond Binary Predictions of Human Drug-Induced Liver Injury (DILI) toward contrasting relative risk potential. Chem Res Toxicol 33(1):223–238. https://doi.org/10.1021/acs.chemrestox.9b00262
Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK et al (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 1:730–741. https://doi.org/10.1002/etc.34
Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, Whitebread S (2012) Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat Rev Drug Discov 11:909
Briggs K, Barber C, Cases M, Marc P, Steger-Hartmann T (2015) Value of Shared preclinical safety studies-the ETOX Database. Toxicol Rep 2:210–221. https://doi.org/10.1016/j.toxrep.2014.12.004
Bull SC, Doig AJ (2015) Properties of protein drug target classes. PLoS One. https://doi.org/10.1371/journal.pone.0117955
Calabrese E (2014) Dose–response: a fundamental concept in toxicology. In Hayes’ principles and methods of toxicology, 6th Edition, pp 89–140. https://doi.org/10.1201/b17359-5
Calabrese EJ, Mattson MP (2017) How does hormesis impact biology, toxicology, and medicine? npj Aging Mech Dis. https://doi.org/10.1038/s41514-017-0013-z
Canepa A, Divino Filho JC, Gutierrez A, Carrea A, Forsberg AM, Nilsson E, Verrina E, Perfumo F, Bergström J (2002) Free amino acids in plasma, red blood cells, polymorphonuclear leukocytes, and muscle in normal and uraemic children. Nephrol Dial Transplant 17(3):413–421. https://doi.org/10.1093/ndt/17.3.413
Carnegie C (2004) Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol 6 Suppl 6 (6): S3–8
Caron G, Kihlberg J, Goetz G, Ratkova E, Poongavanam V, Ermondi G (2021) Steering new drug discovery campaigns: permeability, solubility, and physicochemical properties in the bro5 chemical space. ACS Med Chem Lett 12(1):13–23. https://doi.org/10.1021/acsmedchemlett.0c00581
Cases M, Briggs K, Steger-Hartmann T, Pognan F, Marc P, Kleinöder T, Schwab C, Pastor M, Wichard J, Sanz F (2014) The ETOX data-sharing project to advance in silico drug-induced toxicity prediction. Int J Mol Sci 15(11):21136–21154. https://doi.org/10.3390/ijms151121136
Coady K, Browne P, Embry M, Hill T, Leinala E, Steeger T, Maślankiewicz L, Hutchinson T (2019) When are adverse outcome pathways and associated Assays “Fit for Purpose” for regulatory decision-making and management of chemicals? Integr Environ Assess Manag 15(4):633–647. https://doi.org/10.1002/ieam.4153
eTRANSAFE Comprehensive analysis of correspondence and validity of animal data for human safety (2021) https://etransafe.eu/the-project/about/ Accessed 20 Apr 2021
Corso G, Cristofano A, Sapere N, La Marca G, Angiolillo A, Vitale M, Fratangelo R, Lombardi T, Porcile C, Intrieri M et al (2017) Serum amino acid profiles in normal subjects and in patients with or at risk of Alzheimer dementia. Dement Geriatr Cogn Dis Extra 7(1):143–159. https://doi.org/10.1159/000466688
Dixit VA (2019) A simple model to solve complex drug toxicity problem. Toxicol Res (Camb) 8:157–171. https://doi.org/10.1039/C8TX00261D
Egorova KS, Ananikov VP (2017) Toxicity of metal compounds: knowledge and myths. Organometallics 36(21):4071–4090. https://doi.org/10.1021/acs.organomet.7b00605
FDA’s Predictive Toxicology Roadmap | FDA (2021) https://www.fda.gov/science-research/about-science-research-fda/fdas-predictive-toxicology-roadmap. Accessed 18 Jan 2021
Feldman M, Dickson B (2017) Plasma electrolyte distributions in humans—normal or skewed? Am J Med Sci 354(5):453–457. https://doi.org/10.1016/j.amjms.2017.07.012
Ferrero E, Brachat S, Jenkins JL, Marc P, Skewes-Cox P, Altshuler RC, Keller CG, Kauffmann A, Sassaman EK, Laramie JM et al (2020) Ten simple rules to power drug discovery with data science. PLoS ComputBiol. https://doi.org/10.1371/journal.pcbi.1008126
Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, Haug C (2007) Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol 1(5):695–703. https://doi.org/10.1177/193229680700100513
Giri AK, Ianevski A, Aittokallio T (2019) Genome-wide off-targets of drugs: risks and opportunities. Cell Biol Toxicol. https://doi.org/10.1007/s10565-019-09491-7
Greene N, Pennie W (2015) Computational toxicology, friend or foe? Toxicol Res 13:1159–1172. https://doi.org/10.1039/c5tx00055f
Halappanavar S, Van Den Brule S, Nymark P, Gaté L, Seidel C, Valentino S, Zhernovkov V, Høgh Danielsen P, De Vizcaya A, Wolff H et al (2020) Adverse outcome pathways as a tool for the design of testing strategies to support the safety assessment of emerging advanced materials at the nanoscale. Part Fibre Toxicol 25:16. https://doi.org/10.1186/s12989-020-00344-4
Henkel R, Hoehndorf R, Kacprowski T, Knüpfer C, Liebermeister W, Waltemath D (2018) Notions of smilarity for systems biology models. Brief Bioinform 19(1):77–88. https://doi.org/10.1093/bib/bbw090
Hopkins AL, Keserü GM, Leeson PD, Rees DC, Reynolds CH (2014) The role of ligand efficiency metrics in drug discovery. Nat Rev Drug Discov 13:105
Hughes JD, Blagg J, Price DA, Bailey S, DeCrescenzo GA, Devraj RV, Ellsworth E, Fobian YM, Gibbs ME, Gilles RW et al (2008) Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg Med Chem Lett 18(17):4872–4875. https://doi.org/10.1016/j.bmcl.2008.07.071
Jenkinson S, Schmidt F, Rosenbrier Ribeiro L, Delaunois A, Valentin JP (2020) A practical guide to secondary pharmacology in drug discovery. J Pharmacol Toxicol Methods 105:106869. https://doi.org/10.1016/j.vascn.2020.106869
Kenna JG, Uetrecht J (2018) Do in vitro assays predict drug candidate idiosyncratic drug-induced liver injury risk? Drug Metab Dispos 46(11):1658–1669. https://doi.org/10.1124/dmd.118.082719
Leeson PD (2018) Impact of physicochemical properties on dose and hepatotoxicity of oral drugs. Chem Res Toxicol 31(6):494–505. https://doi.org/10.1021/acs.chemrestox.8b00044
Leeson PD, Young RJ (2015) Molecular property design: does everyone get it? ACS Med Chem Lett 6(7):722–725. https://doi.org/10.1021/acsmedchemlett.5b00157
Maggiora GM (2011) The reductionist paradox: are the laws of chemistry and physics sufficient for the discovery of new drugs. J Comput-Aided Mol Des. https://doi.org/10.1007/s10822-011-9447-8
Muller PY, Milton MN (2012) The determination and Interpretation of the therapeutic index in drug development. Nat Rev Drug Discov 11:751
Nelson DL, Cox MM (2017) Lehninger principles of biochemistry, 7th edn. W.H. Freeman, New York
Pugsley MK, Bekele B, Griessel H, de Korte T, Authier S, Grobler AF, Markgraf CG, Curtis MJ (2020) Twenty years of safety pharmacology model validation and the wider implications of this to drug discovery. J Pharmacol Toxicol Methods 106:912. https://doi.org/10.1016/j.vascn.2020.106912
Richard AM, Huang R, Waidyanatha S, Shinn P, Collins BJ, Thillainadarajah I, Grulke CM, Williams AJ, Lougee RR, Judson RS et al (2021) The Tox21 10K compound library: collaborative chemistry advancing toxicology. Chem Res Toxicol 15:189–216. https://doi.org/10.1021/acs.chemrestox.0c00264
Rivas AM, Mulkey Z, Lado-Abeal J, Yarbrough S (2014) Diagnosing and managing low serum testosterone. In: Baylor Univ. Med. Cent. Proc. 2014, 27(4): 321–324. https://doi.org/10.1080/08998280.2014.11929145
Savulescu J (2020) The six principles, philosophy, and applying human ethics to animals. In: Principles of animal research ethics. Oxford University Press, pp 127–146. https://doi.org/10.1093/med/9780190939120.003.0008
Schneider P, Walters WP, Plowright AT, Sieroka N, Listgarten J, Goodnow RA, Fisher J, Jansen JM, Duca JS, Rush TS et al (2020) Rethinking drug design in the artificial intelligence era. Nat Rev Drug Discov Nat Res 1:353–364. https://doi.org/10.1038/s41573-019-0050-3
Shaw J, Leveridge M, Norling C, Karén J, Molina DM, O’Neill D, Dowling JE, Davey P, Cowan S, Dabrowski M et al (2018) Determining direct binders of the androgen receptor using a high-throughput cellular thermal shift assay. Sci Rep 8(1):163. https://doi.org/10.1038/s41598-017-18650-x
Shultz MD (2019) Two decades under the influence of the rule of five and the changing properties of approved oral drugs. J Med Chem 28:1701–1714. https://doi.org/10.1021/acs.jmedchem.8b00686
Sterling K, Bellabarba D, Newman ES, Brenner MA (1969) Determination of triiodothyronine concentration in human serum. J Clin Invest 48(6):1150–1158. https://doi.org/10.1172/JCI106072
Strech D (2019) Dirnagl U 3Rs missing: animal research without scientific value is unethical. BMJ Open Sci. https://doi.org/10.1136/bmjos-2018-000048
TransQST (2021) Translational quantitative systems toxicology. http://transqst.org/. Accessed 20 Apr 2021
Weaver RJ, Valentin J-P (2019) Today’s challenges to de-risk and predict drug safety in human “Mind-the-Gap. Toxicol Sci 167(2):307–321. https://doi.org/10.1093/toxsci/kfy270
Wenlock MC (2016) Profiling the estimated plasma concentrations of 215 marketed oral drugs. Medchemcomm 7(4):706–719. https://doi.org/10.1039/C5MD00583C
Wolf YI, Katsnelson MI, Koonin EV (2018) Physical foundations of biological complexity. Proc Natl Acad Sci USA 11:E8678–E8687. https://doi.org/10.1073/pnas.1807890115
Funding
No specific funding was received for this work.
Author information
Authors and Affiliations
Contributions
VAD conceptualized the perspective, performed literature search and wrote the article. PS contributed by performing literature search and summarizing the FDA, ICH and OECD guidelines in the context of the perspective.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dixit, V.A., Singh, P. A property-response perspective on modern toxicity assessment and drug toxicity index (DTI). In Silico Pharmacol. 9, 37 (2021). https://doi.org/10.1007/s40203-021-00096-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40203-021-00096-9