Abstract
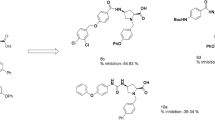
Aspirin (Asp) is one of the most important and ancient member of nonsteroidal anti-inflammatory drugs (NSAID), commonly used in medication of fever, pain and inflammation. It can inhibit the synthesis of prostaglandin by blocking the cyclooxygenase (COX). Attempts have been taken to analyze aspirin together with some of its modified derivatives applying quantum mechanical calculations in order to compare their physicochemical and biochemical properties. Density functional theory (DFT) with B3LYP/6-31G (d, p) basis set has been employed to elucidate their thermal, molecular orbital, equilibrium geometrical properties in gas phase. Molecular docking and nonbonding interactions have been performed against human cyclooxygenase-2 protein 5F1A to investigate the binding affinity and mode(s) of newly designed aspirin derivatives. ADMET prediction has been utilized to compare the absorption, metabolism, and carcinogenic properties of new derivatives with parent drug (Asp). Thermal and geometrical results support the thermochemical stability and equilibrium geometry of all the structures. From the molecular docking simulation, most of the derivatives exhibited better binding affinity than parent drug (Asp) with the receptor protein (5F1A). ADMET prediction disclosed the improved pharmacokinetic properties with lower acute oral toxicity of some derivatives. Based on quantum chemical, molecular docking and ADMET analysis, this investigation can be useful to understand the physicochemical and biochemical/biological activities of Asp and its modified derivatives to search a new antipyretic analgesic drug.





Similar content being viewed by others
Abbreviations
- Asp:
-
Aspirin
- NSAID:
-
Nonsteroidal anti-inflammation drug
- DFT:
-
Density functional theory
- HOMO:
-
Highest occupied molecular orbital
- LUMO:
-
Lowest unoccupied molecular orbital
- MEP:
-
Molecular electrostatic potential
- ADMET:
-
Absorption, distribution, metabolism, excretion, toxicity
References
Accelrys Discovery Studio version 4.1 (2017) Accelrys, San Diego, USA. https://www.3dsbiovia.com/products/collaborative-science/biovia-discoverystudio/requirements/technical-requirements-410.html
Amin ML (2013) P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights. 2013(7):27
Aspirin GR (2001) An ab initio quantum-mechanical study of conformational preferences and of neighboring group interactions. J Org Chem 66(3):771–779
Åqvist J, Medina C, Samuelsson J-E (1994) A new method for predicting binding affinity in computer-aided drug design. Protein Eng Des Sel 7(3):385–391. https://doi.org/10.1093/protein/7.3.385
Awtry EH, Joseph L (2000) Aspirin. Circulation 101(10):1206–1218. https://doi.org/10.1161/01.CIR.101.10.1206
Azam F, Alabdullah NH, Ehmedat HM, Abulifa AR, Taban I, Upadhyayula S (2018) NSAIDs as potential treatment option for preventing amyloid β toxicity in Alzheimer’s disease: an investigation by docking, molecular dynamics, and DFT studies. J Biomol Struct Dyn 36(8):2099–2117
Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al (2009) Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Elsevier
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Boczar M, Wójcik MJ, Szczeponek K, Jamróz D, Zieba A, Kawałek B (2003) Theoretical modeling of infrared spectra of aspirin and its deuterated derivative. Chem Phys 286(1):63–79
Calais J-L (1989) Density-functional theory of atoms and molecules. Parr RG, Yang W. Oxford University Press, New York, Oxford, pp IX+333 pp. Price £45.00. Int. J. Quantum Chem. [Internet]. John Wiley & Sons, Inc. 47(1):101. Available from: https://dx.doi.org/10.1002/qua.560470107
Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B et al (2001) Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 345(25):1809–1817. https://doi.org/10.1056/NEJMoa003199
Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G et al (2012) admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties. J Chem Inf Model 52(11):3099–3105. https://doi.org/10.1021/ci300367a
Cohen N, Benson SW (1993) Estimation of heats of formation of organic compounds by additivity methods. Chem Rev 93(7):2419–2438
Crofford LJ (1997) COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl 49:15–19
Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P et al (2009) Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 10(5):501–507
Dallakyan S, Olson AJ (2015) Small-Molecule library screening by docking with PyRx. In: Hempel JE, Williams CH, Hong CC (eds) Chem. Biol. Methods Protoc. [Internet]. Springer New York, New York, pp 243–50. https://dx.doi.org/10.1007/978-1-4939-2269-7_19
Datt A, Fields D, Larsen SC (2012) An experimental and computational study of the loading and release of aspirin from zeolite HY. J Phys Chem C 116(40):21382–21390
Delano WL (2002) The PyMOL molecular graphics system. De-Lano Scientific, San Carlos, CA, USA. https://www.pymol.org [Internet].; Available from: https://ci.nii.ac.jp/naid/10025409089/en/
El-Shahawy A (2014) DFT cancer energy barrier and spectral studies of aspirin, paracetamol and some analogues. Comput Chem 2(1):6–17
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2016) Gaussian 16. Gaussian, Inc., Wallingford
Garbett NC, Chaires JB (2012) Thermodynamic studies for drug design and screening. Expert Opin Drug Discov 7(4):299–314. https://doi.org/10.1517/17460441.2012.666235
Gleeson MP, Gleeson D (2009) QM/MM calculations in drug discovery: a useful method for studying binding phenomena? J Chem Inf Model 49(3):670–677. https://doi.org/10.1021/ci800419j
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 18(15):2714–2723. https://doi.org/10.1002/elps.1150181505
Husain MA, Rehman SU, Ishqi HM, Sarwar T, Tabish M (2015) Spectroscopic and molecular docking evidence of aspirin and diflunisal binding to DNA: a comparative study. RSC Adv 5(79):64335–64345. https://doi.org/10.1039/C5RA09181K
Insel PA (1996) Analgesic-antipyretic and anti-inlammatory agents and drugs employed in the treatment of gout. The Pharmacological Basic of Therapeutics. pp 617–657. https://ci.nii.ac.jp/naid/10026618116/en/#cit
Juillerat-Jeanneret L, Schmitt F (2007) Chemical modification of therapeutic drugs or drug vector systems to achieve targeted therapy: looking for the grail. Med Res Rev 27(4):574–590
Khan MF, Bin RR, Rashid MA (2015) Computational study of geometry, molecular properties and docking study of aspirin. World J Pharm Res 4:2702–2714
Koohshekan B, Divsalar A, Saiedifar M, Saboury AA, Ghalandari B, Gholamian A et al (2016) Protective efects of aspirin on the function of bovine liver catalase: a spectroscopy and molecular docking study. J Mol Liq 218:8–15. https://doi.org/10.1016/j.molliq.2016.02.022
Kruse H, Goerigk L, Grimme S (2012) Why the standard B3LYP/6–31G* model chemistry should not be used in DFT calculations of molecular thermochemistry: understanding and correcting the problem. J Org Chem 77(23):10824–10834. https://doi.org/10.1021/jo302156p
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789. https://doi.org/10.1103/PhysRevB.37.785
Lewis HD Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE III et al (1983) Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina: results of a Veterans Administration Cooperative Study. N Engl J Med 309(7):396–403
Li X-Q, Andersson TB, Ahlström M, Weidolf L (2004) Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 32(8):821–827
Lien EJ, Guo Z, Li R, Su C (1982) Use of dipole moment as a parameter in drug-receptor interaction and quantitative structure-activity relationship studies. J Pharm Sci 71(6):641–655
Lu Y, Liu Y, Xu Z, Li H, Liu H, Zhu W (2012) Halogen bonding for rational drug design and new drug discovery. Expert Opin Drug Discov 7(5):375–383
Lucido MJ, Orlando BJ, Vecchio AJ, Malkowski MG (2016) Crystal structure of aspirin-acetylated human cyclooxygenase-2: insight into the formation of products with reversed stereochemistry. Biochemistry 55(8):1226–1238. https://doi.org/10.1021/acs.biochem.5b01378
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113(18):6378–6396. https://doi.org/10.1021/jp810292n
Marjan MN, Hamzeh MT, Rahman E, Sadeq V (2014) A computational prospect to aspirin side effects: aspirin and COX-1 interaction analysis based on non-synonymous SNPs. Comput Biol Chem 51:57–62
McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR et al (2018a) Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 379(16):1509–1518
McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R et al (2018b) Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 379(16):1499–1508
Mukherjee D, Nissen SE, Topol EJ (2001) Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 286(8):954–959
Parr RG, Zhou Z (1993) Absolute hardness: unifying concept for identifying shells and subshells in nuclei, atoms, molecules, and metallic clusters. Acc Chem Res 26(5):256–258
Patrignani P, Patrono C (2016) Aspirin and cancer. J Am Coll Cardiol 68(9):967–976
Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci 83(22):8440–8441. https://doi.org/10.1073/pnas.83.22.8440
Pearson RG (1995) The HSAB principle—more quantitative aspects. Inorganica Chim. Acta 240(1):93–98. https://doi.org/10.1016/0020-1693(95)04648-8
Pence HE, Williams A (2010) ChemSpider: an online chemical information resource. J Chem Educ 87(11):1123–1124. https://doi.org/10.1021/ed100697w
Plano D, Karelia DN, Pandey MK, Spallholz JE, Amin S, Sharma AK (2016) Design, synthesis, and biological evaluation of novel selenium (Se-NSAID) molecules as anticancer agents. J. Med. Chem. 59(5):1946–1959
Politzer P, Truhlar DG (2013) Chemical applications of atomic and molecular electrostatic potentials: reactivity, structure, scattering, and energetics of organic, inorganic, and biological systems. Springer Science & Business Media, New York
Richman IB, Owens DK (2017) Aspirin for primary prevention. Med Clin North Am 101(4):713–724. https://doi.org/10.1016/j.mcna.2017.03.004
Ridker PM, Cook NR, Lee I-M, Gordon D, Gaziano JM, Manson JE et al (2005) A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 352(13):1293–1304
Sanguinetti MC, Tristani-Firouzi M (2006) hERG potassium channels and cardiac arrhythmia. Nature 440(7083):463–469
Sarwar MG, Ajami D, Theodorakopoulos G, Petsalakis ID, Rebek J (2013) Amplified halogen bonding in a small space. J Am Chem Soc 135:13672–13675
Saxena A, Balaramnavar VM, Hohlfeld T, Saxena AK (2013) Drug/ drug interaction of common NSAIDs with antiplatelet efect of aspirin in human platelets. Eur J Pharmacol 721(1):215–224. https://doi.org/10.1016/j.ejphar.2013.09.032
Scrocco E, Tomasi J (1973) The electrostatic molecular potential as a tool for the interpretation of molecular properties. New concepts II. Springer, New York, pp 95–170.
Seeliger D, De Groot BL (2010) Conformational transitions upon ligand binding: holo-structure prediction from apo conformations. PLoS Comput Biol 6(1):e1000634
Seshasai SRK, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N et al (2012) Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med 172(3):209–216
Silagy CA, McNeil JJ, Donnan GA, Tonkin AM, Worsam B, Campion K (1993) Adverse effects of low-dose aspirin in a healthy elderly population. Clin Pharmacol Ther 54(1):84–89. https://doi.org/10.1038/clpt.1993.115
Sostres C, Gargallo CJ, Arroyo MT, Lanas A (2010) Adverse effects of non-steroidal anti-inlammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol 24(2):121–132. https://doi.org/10.1016/j.bpg.2009.11.005
Thun MJ, Jacobs EJ, Patrono C (2012) The role of aspirin in cancer prevention. Nat Rev Clin Oncol 9(5):259
Thun MJ, Namboodiri MM, Heath CW Jr (1991) Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 325(23):1593–1596
Uzzaman M, Hoque MJ. Uzzaman M, Hoque MJ (2018) Physiochemical, molecular docking, and pharmacokinetic studies of Naproxen and its modified derivatives based on DFT. Int J Sci Res Manag 6:6(09 SE-Chemistry). 10.18535/ijsrm/v6i9.c01. Available from: https://ijsrm.in/index.php/ijsrm/article/view/1789
Uzzaman M, Shawon J, Siddique ZA (2019) Molecular docking, dynamics simulation and ADMET prediction of Acetaminophen and its modified derivatives based on quantum calculations. SN Appl Sci 1(11):1437. https://doi.org/10.1007/s42452-019-1442-z
Uzzaman M, Uddin MN (2019) Optimization of structures, biochemical properties of ketorolac and its degradation products based on computational studies. DARU J Pharm Sci 27(1):71–82. https://doi.org/10.1007/s40199-019-00243-w
Wade RC, Goodford PJ (1989) The role of hydrogen-bonds in drug binding. Prog. Clin. Biol. Res. 289:433–444. Available from: https://europepmc.org/abstract/MED/2726808
Wade RC, Goodford PJ (1989) The role of hydrogen-bonds in drug binding. Prog Clin Biol Res 289:433–444
Walum E (1998) Acute oral toxicity. Environ Health Perspect 106:497–503. https://doi.org/10.1289/ehp.98106497
Acknowledgements
Authors are thankful to department of chemistry, University of Chittagong for optimization support and Mohammad Jabedul Hoque, Department of Optoelectronics and Nanostructure Science, Shizuoka University, Japan for his valuable suggestions.
Funding
This research not received any fund.
Author information
Authors and Affiliations
Contributions
MU designed the project. TM perform all calculation and data collection. MU and TM wrote the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uzzaman, M., Mahmud, T. Structural modification of aspirin to design a new potential cyclooxygenase (COX-2) inhibitors. In Silico Pharmacol. 8, 1 (2020). https://doi.org/10.1007/s40203-020-0053-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40203-020-0053-0