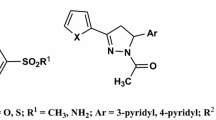
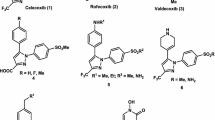
This work was aimed at synthesising and characterising five new conjugates of aspirin with aromatic amino acids and testing them in vitro as potential selective cyclooxygenase 2 (COX-2) inhibitors. The conjugates were synthesized by a 3-step approach and successfully characterized by analyzing their 1H and 13C NMR and optical spectra. The in vitro activity was tested against COX-1 and COX-2 using celecoxib and aspirin as control drugs, and the ADME properties were investigated through SwissADME web tool. All the synthesized conjugates were less active than aspirin on COX-1 and more active on COX-2, and 3 of them (compounds Y1, Y3 and Y5) showed activity and selectivity comparable to those of celecoxib. SwissADME predicted that the conjugates would have high gastrointestinal absorption and better synthetic accessibility than celecoxib. They did not score any violation from the drug-likeness filters as well. Being composed of well-known molecules, these conjugates offer the advantage of being safe and easy to synthesize potential selective inhibitors of COX-2.



Similar content being viewed by others
References
H. Svanström, M. Lund, M. Melbye, and B. Pasternak, Pharmacoepidemiol. Drug Saf., 27(8), 885 – 893 (2018).
G. Garcia-Rayado, M. Navarro, and A. Lanas, Expert Rev. Clin. Pharmacol., 11(10), 1031 – 1043 (2018).
I. Bjarnason, C. Scarpignato, E. Holmgren, et al., Gastroenterology, 154(3), 500 – 514 (2018).
A. A. Elhenawy, L. M. Al-Harbi, G. O. Moustafa, et al., Drug Des. Devel. Ther., 13, 1773 – 1790 (2019).
M. H. M. Jasim, M. N. Abed, M. E. Qazzaz, et al., Trop. J. Pharm. Res., 30(3), 579–583 (2021).
Y. F. Mustafa, R. R. Khalil, E. T. Mohammed, et al., Arch. Razi Inst., 76(5), 1297–1305 (2021).
Y. F. Mustafa, J. Glob. Pharma Technol., 11(9), 1 – 10 (2019).
S. Naik, G. Bhattacharjya, B. Talukdar, and B. K. Patel, Eur. J. Org. Chem., No. 6, 1254 – 1260 (2004).
M. A. Abdelgawad, R. B. Bakr, A. O. El-Gendy, et al., Future Med. Chem., 9(16), 1899 – 1912 (2017).
A. Daina, O. Michielin, and V. Zoete, Sci. Rep., 7(1), 42717 (2017).
Y. F. Mustafa and N. A. Mohammed, Biochem. Cell. Arch., 21(Supp 1), 1991 – 1999 (2021).
Y. F. Mustafa, R. R. Khalil, and E. T. Mohammed, Egypt. J. Chem., 64(7), 3711 – 3716 (2021).
M. K. Oglah, M. K. Bashir, Y. F. Mustafa, et al., Syst. Rev. Pharm., 11(6), 717 – 725 (2020).
K. M. Mirabito Colafella, R. I. Neuman, W. Visser, et al., Basic Clin. Pharmacol. Toxicol., 127(2), 132–141 (2020).
P. L. McCormack, Drugs, 71(18), 2457–2489 (2011).
J. L. Wang, D. Limburg, M. J. Graneto, et al., Bioorg. Med. Chem. Lett., 20(23), 7159–7163 (2010).
C. A. Lipinski, F. Lombardo, B. W. Dominy, and P. J. Feeney, Adv. Drug Deliv. Rev., 46(1 – 3), 3 – 26 (2001).
A. K. Ghose, V. N. Viswanadhan, and J. J. Wendoloski, J. Comb. Chem., 1(1), 55 – 68 (1999).
D. F. Veber, S. R. Johnson, H.-Y. Cheng, et al., J. Med. Chem., 45(12), 2615 – 2623 (2002).
M. Alfahad, S. S. Ismael, M. H. M. Jasim, et al., Lat. Am. J. Pharm., 40(12), 2914 – 2919 (2021).
P. Ertl and A. Schuffenhauer, J. Cheminform., 1(1), 1 – 11 (2009).
E. M. Ahmed, M. S. A. Hassan, A. A. El-Malah, and A. E. Kassab, Bioorg. Chem., 95(2020), 103497 (2020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jasim, M.H.M., Alfahad, M., Al-Dabbagh, B.M. et al. Synthesis, Characterization, ADME Study and In-Vitro Anti-Inflammatory Activity of Aspirin Amino Acid Conjugates. Pharm Chem J 57, 243–249 (2023). https://doi.org/10.1007/s11094-023-02874-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02874-5