Abstract
Background
Diabetes mellitus (DM) has continued to raise concern globally and Curculigo pilosa (CP) is used for its treatment and management in folkloric medicine. In this study, the in vitro antioxidant abilities of CP and the effects of CP-supplemented diets on blood sugar, lipid metabolism, oxidative stress and key carbohydrate metabolizing enzymes in streptozotocin (STZ)-induced diabetic rats were investigated.
Methods
Polyphenol contents (total phenolic and total flavonoid) and antioxidant ability of different extracts of CP were determined in vitro. Diabetes mellitus were stimulated in healthy rats by single intraperitoneal administration of 50 mg/kg streptozotocin and it was confirmed by elevated blood glucose level after 3 days. Thirty six rats were distributed into six groups of six rats each and diabetic rats were fed with 5 and 10% CP-supplemented diet for 21 days. Thereafter, the effects of the dietary regimen were evaluated on blood glucose, body weight, hepatic carbohydrate metabolizing enzymes, lipid profile, oxidative stress markers, serum markers of hepatic and renal damages and histopathology studies.
Results
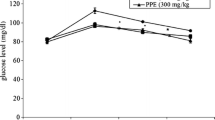
Different extracts of CP contained polyphenol contents and exhibited antioxidant properties in different models used. Diabetic rats showed elevated level of blood glucose and body weight loss. Treatment of diabetic rats with CP-supplemented diet significantly (p < 0.05) lowered the blood glucose and improved body weight loss. Also, the treatment with the CP-supplemented diet significantly (p < 0.05) enhanced the activities of hepatic glycolytic (hexokinase and glucose-6-dehydrogenase) and lowered the gluconeogenic (fructose 1, 6 biphosphatase and glucose-6-phosphatase) enzymes in diabetic rats. The lipid profile, oxidative stress markers and serum markers of hepatic and renal damages were significantly (p < 0.05) restored to near normalcy in the diabetic rats. Histopathological slides also showed improvements in pancreas and hepatic tissues of diabetic rats treated with CP-supplemented diet.
Conclusion
Data obtained in this study suggested that CP-supplemented diet could be used as dietary regimen in the management of DM.








Similar content being viewed by others
References
Cam ME, Hazar-Yavuz AN, Yildiza S, Ertas B, Adakul BA, Taskin T, Alan S, Kabasakal L. The methanolic extract of Thymus praecox subsp. skorpiliivar. skorpiliirestores glucose homeostasis, ameliorates insulin resistance and improvespancreatic β-cell function on streptozotocin/nicotinamide-induced type 2diabetic rats Journal Ethnopharmacol 2019;(231): 29–38.
International Diabetes Federation the IDF Diabetes Atlas, Ninth Edition; 2019.
Levinthal GN, Tavill AS. Liver disease and diabetes mellitus. Clin Diabetes. 1999;17(2):1–20.
Guven A, Yavuz O, Cam M, Ercan F, Bukan N, Comunoglu C, et al. Effects of melatonin on streptozotocin-induced diabetic liver injury in rats. ActaHistochem. 2006;108:85–93. https://doi.org/10.1016/j.acthis.2006.03.005.
Mohamed J, Nafizah AHN, Zariyantey AH, Budin SB. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation Sultan QaboosUniver. Med J. 2016;16(2):132–41.
Karigidi KO, Olaiya CO, Antidiabetic activity of corn steep liquor extract of Curculigo pilosa and its solvent fractions in streptozotocin-induced diabetic rats, J TraditiComplemen Med https://doi.org/10.1016/j.jtcme.2019.06.005, 2019.
Cicero LTC, Yenshou L, Arlene PB, Yi-Ching C, Shao-Chih C, Wen-Chin Y.Herbal Therapies for Type 2 Diabetes Mellitus: Chemistry, Biology, and Potential Application of Selected Plants and Compounds Evidence-Based ComplemenAltern Med Volume 2013 |Article ID 378657 | 33 pages | 10.1155 /2013/378657.
Kasole R, Martin HD, Kimiywe J. Traditional Medicine and Its Role in the Management of Diabetes Mellitus: (Patients’ and Herbalists’ Perspectives Evidence-Based ComplemenAltern Med Volume 2019, Article ID 2835691, 12 pages. https://doi.org/10.1155/2019/2835691.
Nasri H, Shirzad H, Baradaran A, Rafieian-kopaei M. Antioxidant plants and diabetes mellitus. J Res Med Sci Off J Isfahan Univ Med Sci. 2015;20:491–502.
Sofidiya MO, Oduwole B, Bamgbade E, Odukoya O, Adenekan S. Nutritional composition and antioxidant activities of Curculigo pilosa (Hypoxidaceae) rhizome African. J Biotechnol. 2011;10(75):17275–81.
Nie Y, Dong X, He Y, Yuan T, Han T, Rahman K, et al. Medicinal plants of genus Curculigo: traditional uses and a phytochemical and ethnopharmacological review. J Ethnopharmacol. 2013;147:547–63.
Gbadamosi IT, Egunyomi A. Phytochemical screening and invitro anticandidal activity of extracts and essential oil of Curculigo pilosa (Schum and Thonn) Engl. Hypoxidaceae Afr J Biotechnol. 2010;9(8):1236–40.
Olaiya CO, Karigidi KO. Hypoglycemic effects of corn steep liquor extracts in Streptozotocin-induced diabetic rats. Int J Biochem Res Rev. 2016;13(2):1–8.
Adefegha SA, Oyeleye SI, Oboh G. African crocus (Curculigo pilosa) and wonderful kola (Buchholziacoriacea) seeds modulate critical enzymes relevant to erectile dysfunction and oxidative stress. J Complement Integrat Med 2018; DOI: https://doi.org/10.1515/jcim-2016-0159, 15.
Karigidi KO, Olaiya CO. In vitro Antidiabetic, antioxidant and anti-lipid peroxidative activities of corn steep liquor extracts of Curculigopilosa and its solvent fractions, J herbs. Spic Med Plants. 2019;25(4):377–88.
Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–6.
Park Y-S, Jung S-T, Kang S-G, Heo BK, Arancibia-Avila P, Toledo F, et al. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107:640–8.
Oyaizu M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15.
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;26(9):337–41.
Gyamfi M, Yonamine M, Aniya Y. Free radical scavenging action of medicinal herbs from Ghana: Thonningia sanguine on experimentally induced liver injuries. Gen Pharmacol. 1999;32(6):661–7.
Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–6.
Mondal S, Chakraborty G, Gupta M, Muzumdar U. In vitro antioxidant activity of Diospyros malabarica kostel bark. Ind J Experimen Biol. 2006;44:39–44.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic Biol Med. 1999;26(9–10):1231–7.
Oyetayo FL, Akomolafe SF, Odeniyi IA. Effects of dietary supplementation of Chrysophyllum albidum fruit pulp powder on some biochemical parameters in a type 2 diabetes rat model. Vegetos. 2019;32:190–9. https://doi.org/10.1007/s42535-019-00022-7.
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington DC: National Academies Press (US); 2011.
Adedara IA, Awogbindin IO, Anamelechi JP, Farombi EO. Garcinia kola seed ameliorates renal, hepatic, and testicular oxidative damage in streptozotocin-induced diabetic rats.Pharmaceutl biol. 2014:1–10 DOI: https://doi.org/10.3109/13880209.2014.937504
Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with BCG. ClinChimActa. 1971;31:87–96.
Bartels H, Bohmer M. In vitro determination of Creatinine and urea. Clin Chem. 1972;2:37–193.
Weatherburn MW. Colorimetric methods for serum urea determination. Anal Chem. 1967;39:971–4.
Allain GC, Poon LS, Chan CS, Richmond W. Quantitative determination of cholesterol using enzymatic colorimetric method. Clin Chem. 1974;20:470–5.
Jendrassik L, Grof P. In vitro determination of total and direct bilirubin. Biochemica. 1938;297:81.
Jacobs NJ, Van Denmark PJ. Determination of triglycerides. Arch BiochemBiophys. 1960;88:250–5.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamicoxaloacetic and glutamicpyruvictransaminases. Am J Clin Pathol. 1957;28:56–63.
Rec GSCC (DGKC). Colorimetric method for serum alkaline phosphatase determination. J Clin Chem ClinBiochem. 1972;10:182.
Ikewuchi CJ, Ikewuchi CC. Alteration of plasma lipid profiles and atherogenic indices by Stachytarphetajamaicensis L (Vahl). Boikemistri. 2009;21:71–7.
Takasaki Y. Serum lipid levels and factors affecting atherogenic index in Japanese children. J PhysiolAnthropol Appl Hum Sci. 2005;24:511–5.
Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–66.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Green LC, Wagner DA, Glogowski J, Skiper PL, Wishnock JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8.
Brandstrup N, Kirk JE, Bruni C. The hexokinase and phosphogluco isomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol. 1957;12:166–71.
Ellis HA, Kirkman HN. A colorimetric method for assay of erythrocytic glucose 6-phosphate dehydrogenase. Proc Soc Exp Biol Med. 1961;106:607–9.
Koide H, Oda T. Pathological occurrence of glucose 6-phosphatase in serum in liver diseases. ClinChimActa. 1959;4:554–61.
Gancedo JM, Gancedo C. Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Microbiol. 1971;76:132–8.
Avwioro OG. Histochemistry and tissue pathology, principle and techniques. Nigeria: Claverianum press; 2010.
Abshirini M, Mahaki B, Bagheri F, Siassi F, Koohdani F, Sotoudeh G. Higher intake of phytochemical-rich foods is inversely related to prediabetes: a case-control study. Int J Prev Med. 2018;9:64.
Wang PY, Fang JC, Gao ZH, Zhang C, Xie SY. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. J Diabetes Investig. 2016;7:56–69.
Zhang Y-J, Gan R-Y, Li S, Zhou Y, Li A-N. Dong-Ping Xu and Hua-Bin Li Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases Molecules. 2015;20:21138–56. https://doi.org/10.3390/molecules201219753.
Lui RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. The American J ClinNutrit. 2003;78(3):517S–20S. https://doi.org/10.1093/ajcn/78.3.517S.
Kwon YI, Apostolidis E, Kim YC, Shetty K. Health benefits of traditional corn, beans and pumpkin: in vitro studies for hyperglycemia and hypertension management. J Med Food. 2007;10:266–75.
Karigidi KO, Olaiya CO.Curculigo pilosa mitigates against oxidative stress and structural derangements in pancreas and kidney of streptozotocin-induced diabetic rats Journal of Complementary and Integrative Medicine 2020; 20190217 https://doi.org/10.1515/jcim-2019-0217.
Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK. Kong L et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review J TraditComplem Med. 2018;8:361–76.
Wahba NS, Shaban SF, Asmaa A, Kattaia A, Kandeel SA. Efficacy of zinc oxide nanoparticles in attenuating pancreatic damage in a rat model of streptozotocin-induced diabetes. UltrastructPathol, 2016. 2016;40(6):358–73. https://doi.org/10.1080/01913123.2016.1246499.
Erejuwa OO, Nwobodo NN, Akpan JL, Okorie UA, Ezeonu CT, Ezeokpo BC, et al. Nigerian honey ameliorates hyperglycemia and dyslipidemia in Alloxan-induced diabetic rats. Nutrients. 2016;8:95.
Subbiah R, Kasiappan R, Karuran S, Sorimuthu S. Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin and Exp Pharmacol Physiol. 2006;33:232–7.
Nesto RW. Beyond low-density lipoprotein: addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome, am J Cardiovasc. Drugs. 2005;5:379–87.
Li W, Wang G, Lu X, Jiang Y, Xu L, Zhao X. Lycopene ameliorates renal function in rats with streptozotocin-induced diabetes. Int J Clin Exp Pathol. 2014;7(8):5008–15.
Halliwell B. Albumin: an important extracellular antioxidant? Biochem Pharmacol. 1988;37:569–71.
Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. 2002;55:820–9.
Zhang Y, Lu X, Hong J, Chao M, Gu W, Wang W, et al. Positive correlations of liver enzymes with metabolic syndrome including insulin resistance in newly diagnosed type 2 diabetes mellitus. Endocr. 2010;38:181–7.
Succurro E, Arturi F, Grembiale A, Iorio F, Fiorentino TV, Andreozzi F, et al. One-hour post-load plasma glucose levels are associated with elevated liver enzymes. Nutr Metab Cardiovasc Dis. 2011;21:713–8.
Han N, Kyaw-Soe HH, Htet A. Determinants of Abnormal Liver Function Tests in Diabetes Patients in Myanmar. Internat J. Diab Res. 2012;1(3):36–41.
Chatila R, West AB. Hepatomegaly and abnormal liver tests due to glycogenesis in adults with diabetes. Med. 1996;75(6):327–33.
Soliman AM. Potential impact of Paracentrotuslividus extract on diabetic rat models induced by high fat diet/streptozotocin. The J Bas Appl Zool. 2016;77:8–20.
Chan KH, O'Connell RL, Sullivan DR, et al. Plasma total bilirubin levels predict amputation events in type 2 diabetes mellitus: the Fenofibrate intervention and event lowering in diabetes (FIELD) study. Diabetologia. 2013;56(4):724–36. https://doi.org/10.1007/s00125-012-2818-4.
Breimer LH, Mikhailidis DP. Is bilirubin a marker of vascular disease and/or cancer and is it a potential therapeutic target? CurrPharm Des. 2011;17:3644–55.
Kimm H, Yun JE, Jo J, Jee SH. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke; J cerebcirculat. 2009;40:3422–7.
Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. Jama. 2007;298:1398–400.
Xie Z, Zeng X, Li X, Wu B, Shen G, Wu Q, et al. Curcumin attenuates oxidative stress in liver in type 1 diabetic rats. Open Life Sci. 2017;12:452–9.
Murunga AN, Miruka DO, Driver C, Nkomo FS, Cobongela SZZ, Owira PMO. Grapefruit derived flavonoid Naringin improves ketoacidosis and lipid peroxidation in type 1 diabetes rat model. PLoS ONE. 2016;11(4):e0153241. https://doi.org/10.1371/journal.pone.0153241.
Irudayaraj SS, Christudas S, Antony S, Duraipandiyan V, Abdullah AN, Ignacimuthu S. Protective effects of Ficuscarica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and β-cells in type 2 diabetic rats. Pharma Biol. 2017;55(1):1074–81. https://doi.org/10.1080/13880209.2017.1279671.
Hikino H, Kobayashi M, Suzuki Y, Konno C. Mechanisms of hypoglycemic activity of aconitan a, a glycan from Aconitum carmichaeli roots. J Ethnopharmacol. 1989;25:295–304.
Sundaram R, Naresh R, Ranadevan R, Shanthi P, Sachdanandam P. Effect of iridoidglucoside on streptozotocin induced diabetic rats and its role in regulating carbohydrate metabolic enzymes. Eur J Pharmacol. 2012;674:460–7.
Sankaranarayanan C, Ramajayam N, Pachaiappan P. Ameliorating effect of berbamine on hepatic key enzymes of carbohydrate metabolism in high-fat diet and streptozotocin induced type 2 diabetic rats. Biomed Pharmacother. 2018;103:539–45.
Saravanan G, Ponmurugan P, Deepa MA, Senthilkumar B. Modulatory effects of Diosgenin on attenuating the key enzymes activities of carbohydrate metabolism and glycogen content in Streptozotocin-induced diabetic rats. Can J Diabet. 2014;38:409–14.
Basha RH, Sankaranarayanan C. β-Caryophyllene, a natural sesquiterpene, modulates carbohydrate metabolism in streptozotocin-induced diabetic rats. ActaHistochemica. 2014;116:1469–79.
Kosuru R, Singh S. Pterostilbene ameliorates insulin sensitivity, glycemic control and oxidative stress in fructose-fed diabetic rats. Life Sci. 2017;182:112–21. https://doi.org/10.1016/j.lfs.2017.06.015.
Gang L, Guangxiang L, Yanfeng H, Fangfang T, Zhenhua W, Yourui S, Chengjun M, Honglun W Polyphenol Stilbenes from Fenugreek (Trigonella foenum graecum L.) Seeds Improve Insulin Sensitivity and MitochondrialFunction in 3T3-L1. Adipocytes. Oxid Med CellulLongev. 2018; https://doi.org/10.1155/2018 /7634362.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karigidi, K.O., Akintimehin, E.S., Omoboyowa, D.A. et al. Effect of Curculigo pilosa supplemented diet on blood sugar, lipid metabolism, hepatic oxidative stress and carbohydrate metabolism enzymes in streptozotocin-induced diabetic rats. J Diabetes Metab Disord 19, 1173–1184 (2020). https://doi.org/10.1007/s40200-020-00618-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00618-w