Abstract
Purpose of Review
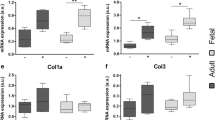
Injured skin in the mammalian fetus can heal regeneratively due to the ability of fetal fibroblasts to effectively reorganize the extracellular matrix (ECM). This process occurs without fetal fibroblasts differentiating into highly contractile myofibroblasts which cause scarring and fibrosis in adult wounds. Here, we provide a brief review of fetal wound healing and the evidence supporting a unique contractile phenotype in fetal fibroblasts. Furthermore, we discuss the biomechanical role of the ECM in driving myofibroblast differentiation in wound healing and the implications for new clinical modalities based on the biophysical properties of fetal fibroblasts.
Recent Findings
We and others have found that fetal fibroblasts are refractory to the environmental stimuli necessary for myofibroblast differentiation in adult wound healing including mechanical stress.
Summary
Understanding the biomechanical mechanisms that regulate the contractile phenotype of fetal fibroblasts may unlock new avenues for anti-scarring therapies that target myofibroblast differentiation of adult fibroblasts.


Similar content being viewed by others
References
Papers of particular interest with historical significance have been highlighted as: • Of importance
Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71. doi:10.1111/j.1524-475X.2009.00543.x.
Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1–2):113–25. doi:10.2119/molmed.2009.00153.
Cass DL, Bullard KM, Sylvester KG, Yang EY, Longaker MT, Adzick NS. Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg. 1997;32(3):411–5.
Burrington JD. Wound healing in the fetal lamb. J Pediatr Surg. 1971;6(5):523–8.
Dohar JE, Klein EC, Betsch JL, Hebda PA. Fetal airway wound repair: a new frontier. Arch Otolaryngol Head Neck Surg. 1998;124(1):25–9.
Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143–52.
Wilgus TA. Regenerative healing in fetal skin: a review of the literature. Ostomy Wound Manage. 2007;53(6):16–31. quiz 2-3
• Lorenz HP, Lin RY, Longaker MT, Whitby DJ, Adzick NS. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg. 1995;96(6):1251–9. discussion 60-1. This study was critical for establishing the role of human dermal fetal fibroblasts in scarless healing in vivo .
Knight KR, Lepore DA, Horne RS, Ritz M, Hurley JV, Kumta S, et al. Collagen content of uninjured skin and scar tissue in foetal and adult sheep. Int J Exp Pathol. 1993;74(6):583–91.
Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43(1):146–55. doi:10.1016/j.jbiomech.2009.09.020.
Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, et al. Mechanotransduction and fibrosis. J Biomech. 2014;47(9):1997–2005. doi:10.1016/j.jbiomech.2014.03.031.
Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–11.
Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142(3):873–81.
Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, Adzick NS. Scarless wound repair: a human fetal skin model. Development. 1992;114(1):253–9.
Haynes JH, Krummel TM, Schatzki PF, Dunn JD, Flood LC, Cohen IK, et al. Histology of the open fetal rabbit wound. Surg Forum. 1989;40:558–60.
Krummel TM, Ehrlich HP, Nelson JM, Michna BA, Thomas BL, Haynes JH, et al. In vitro and in vivo analysis of the inability of fetal rabbit wounds to contract. Wound Repair Regen. 1993;1(1):15–21. doi:10.1046/j.1524-475X.1993.10106.x.
Dostal GH, Gamelli RL. Fetal wound healing. Surg Gynecol Obstet. 1993;176(3):299–306.
Hallock GG, Rice DC, Merkel JR, DiPaolo BR. Analysis of collagen content in the fetal wound. Ann Plast Surg. 1988;21(4):310–5.
Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25(1):63–8. discussion 8-9
Julia MV, Albert A, Morales L, Miro D, Sancho MA, Garcia X. Wound healing in the fetal period: the resistance of the scar to rupture. J Pediatr Surg. 1993;28(11):1458–62.
Lovvorn HN 3rd, Cheung DT, Nimni ME, Perelman N, Estes JM, Adzick NS. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg. 1999;34(1):218–23.
Gharaee-Kermani M, Phan SH. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des. 2001;7(11):1083–103.
Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi:10.3410/B2-78.
Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, Adzick NS. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg. 1994;219(1):65–72.
Wider TM, Yager JS, Rittenberg T, Hugo NE, Ehrlich HP. The inhibition of fibroblast-populated collagen lattice contraction by human amniotic fluid: a chronologic examination. Plast Reconstr Surg. 1993;91(7):1287–93.
Hohlfeld J, de Buys RA, Hirt-Burri N, Chaubert P, Gerber S, Scaletta C, et al. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366(9488):840–2. doi:10.1016/S0140-6736(05)67107-3.
Lo DD, Zimmermann AS, Nauta A, Longaker MT, Lorenz HP. Scarless fetal skin wound healing update. Birth Defects Res C Embryo Today. 2012;96(3):237–47. doi:10.1002/bdrc.21018.
Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol. 2012;2012:698034. doi:10.5402/2012/698034.
Hu MS, Maan ZN, Wu JC, Rennert RC, Hong WX, Lai TS, et al. Tissue engineering and regenerative repair in wound healing. Ann Biomed Eng. 2014;42(7):1494–507. doi:10.1007/s10439-014-1010-z.
Walraven M, Gouverneur M, Middelkoop E, Beelen RH, Ulrich MM. Altered TGF-beta signaling in fetal fibroblasts: what is known about the underlying mechanisms? Wound Repair Regen. 2014;22(1):3–13. doi:10.1111/wrr.12098.
Gosiewska A, Yi CF, Brown LJ, Cullen B, Silcock D, Geesin JC. Differential expression and regulation of extracellular matrix-associated genes in fetal and neonatal fibroblasts. Wound Repair Regen. 2001;9(3):213–22.
Broker BJ, Chakrabarti R, Blynman T, Roesler J, Wang MB, Srivatsan ES. Comparison of growth factor expression in fetal and adult fibroblasts: a preliminary report. Arch Otolaryngol Head Neck Surg. 1999;125(6):676–80.
Moulin V, Tam BY, Castilloux G, Auger FA, O'Connor-McCourt MD, Philip A, et al. Fetal and adult human skin fibroblasts display intrinsic differences in contractile capacity. J Cell Physiol. 2001;188(2):211–22. doi:10.1002/jcp.1110.
Lanning DA, Diegelmann RF, Yager DR, Wallace ML, Bagwell CE, Haynes JH. Myofibroblast induction with transforming growth factor-beta1 and -beta3 in cutaneous fetal excisional wounds. J Pediatr Surg. 2000;35(2):183–7. discussion 7-8
Colwell AS, Krummel TM, Longaker MT, Lorenz HP. Fetal and adult fibroblasts have similar TGF-beta-mediated, Smad-dependent signaling pathways. Plast Reconstr Surg. 2006;117(7):2277–83. doi:10.1097/01.prs.0000224299.16523.76.
Moulin V, Plamondon M. Differential expression of collagen integrin receptor on fetal vs. adult skin fibroblasts: implication in wound contraction during healing. Br J Dermatol. 2002;147(5):886–92.
Coleman C, Tuan TL, Buckley S, Anderson KD, Warburton D. Contractility, transforming growth factor-beta, and plasmin in fetal skin fibroblasts: role in scarless wound healing. Pediatr Res. 1998;43(3):403–9. doi:10.1203/00006450-199803000-00016.
Parekh A, Sandulache VC, Lieb AS, Dohar JE, Hebda PA. Differential regulation of free-floating collagen gel contraction by human fetal and adult dermal fibroblasts in response to prostaglandin E2 mediated by an EP2/cAMP-dependent mechanism. Wound Repair Regen. 2007;15(3):390–8. doi:10.1111/j.1524-475X.2007.00241.x.
Ehrlich HP, Rajaratnam JB. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell. 1990;22(4):407–17.
Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124(4):401–4.
Sandulache VC, Parekh A, Li-Korotky HS, Dohar JE, Hebda PA. Prostaglandin E2 differentially modulates human fetal and adult dermal fibroblast migration and contraction: implication for wound healing. Wound Repair Regen. 2006;14(5):633–43. doi:10.1111/j.1743-6109.2006.00156.x.
Sandulache VC, Parekh A, Dohar JE, Hebda PA. Fetal dermal fibroblasts retain a hyperactive migratory and contractile phenotype under 2-and 3-dimensional constraints compared to normal adult fibroblasts. Tissue Eng. 2007;13(11):2791–801. doi:10.1089/ten.2006.0412.
Parekh A, Sandulache VC, Singh T, Cetin S, Sacks MS, Dohar JE, et al. Prostaglandin E2 differentially regulates contraction and structural reorganization of anchored collagen gels by human adult and fetal dermal fibroblasts. Wound Repair Regen. 2009;17(1):88–98. doi:10.1111/j.1524-475X.2008.00445.x.
Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature reviews. 2009;10(1):63–73. doi:10.1038/nrm2597.
• Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159(3):1009–20. doi:10.1016/S0002-9440(10)61776-2. This study was critical for establishing that mechanical tension in granulation tissue regulated myofibroblast activity .
Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1—an intimate relationship. Eur J Cell Biol. 2008;87(8–9):601–15. doi:10.1016/j.ejcb.2008.01.012.
Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154(3):871–82.
Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21(12):3250–61. doi:10.1096/fj.07-8218com.
Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011;194(1):25–37. doi:10.1159/000322399.
Lin GL, Cohen DM, Desai RA, Breckenridge MT, Gao L, Humphries MJ, et al. Activation of beta 1 but not beta 3 integrin increases cell traction forces. FEBS Lett. 2013;587(6):763–9. doi:10.1016/j.febslet.2013.01.068.
Kim JK, Xu Y, Xu X, Keene DR, Gurusiddappa S, Liang X, et al. A novel binding site in collagen type III for integrins alpha1beta1 and alpha2beta1. J Biol Chem. 2005;280(37):32512–20. doi:10.1074/jbc.M502431200.
Yang L, Tsai CM, Hsieh AH, Lin VS, Akeson WH, Sung KL. Adhesion strength differential of human ligament fibroblasts to collagen types I and III. J Orthop Res. 1999;17(5):755–62. doi:10.1002/jor.1100170521.
Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proc Natl Acad Sci U S A. 2009;106(38):16245–50. doi:10.1073/pnas.0902818106.
Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014;35(1):71–82. doi:10.1016/j.biomaterials.2013.09.066.
Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 2004;12(2):134–47. doi:10.1111/j.1067-1927.2004.012208.x.
Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13(5):264–9.
Huang C, Akaishi S, Ogawa R. Mechanosignaling pathways in cutaneous scarring. Arch Dermatol Res. 2012;304(8):589–97. doi:10.1007/s00403-012-1278-5.
Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr. 2012;24(3):371–8. doi:10.1097/MOP.0b013e3283535790.
Ryu JH, Daniels CE. Advances in the management of idiopathic pulmonary fibrosis. F1000 Med Rep. 2010;2:28. doi:10.3410/M2-28.
Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2011;18(1):148–52. doi:10.1038/nm.2574.
Paterno J, Vial IN, Wong VW, Rustad KC, Sorkin M, Shi Y, et al. Akt-mediated mechanotransduction in murine fibroblasts during hypertrophic scar formation. Wound Repair Regen. 2011;19(1):49–58. doi:10.1111/j.1524-475X.2010.00643.x.
Rustad KC, Wong VW, Gurtner GC. The role of focal adhesion complexes in fibroblast mechanotransduction during scar formation. Differentiation. 2013;86(3):87–91. doi:10.1016/j.diff.2013.02.003.
Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–17. doi:10.1038/nm.2208.
Gurtner GC, Dauskardt RH, Wong VW, Bhatt KA, Wu K, Vial IN, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254(2):217–25. doi:10.1097/SLA.0b013e318220b159.
Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. doi:10.1038/nature07039.
Hebda PA, Dohar JE. Transplanted fetal fibroblasts: survival and distribution over time in normal adult dermis compared with autogenic, allogenic, and xenogenic adult fibroblasts. Otolaryngol Head Neck Surg. 1999;121(3):245–51. doi:10.1016/S0194-5998(99)70179-8.
Sandulache VC, Zhou Z, Sherman A, Dohar JE, Hebda PA. Impact of transplanted fibroblasts on rabbit skin wounds. Arch Otolaryngol Head Neck Surg. 2003;129(3):345–50.
Sandulache VC, Dohar JE, Hebda PA. Fibroblast transplantation in the airway: implications for subglottic stenosis. Arch Otolaryngol Head Neck Surg. 2005;131(12):1090–6. doi:10.1001/archotol.131.12.1090.
Sandulache VC, Dohar JE, Hebda PA. Adult-fetal fibroblast interactions: effects on cell migration and implications for cell transplantation. Cell Transplant. 2005;14(5):331–7.
Wadman M. The truth about fetal tissue research. Nature. 2015;528(7581):178–81. doi:10.1038/528178a.
Acknowledgements
We acknowledge the support provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R03AR066875 to AP. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Wound Healing and Tissue Repair
Rights and permissions
About this article
Cite this article
Parekh, A., Hebda, P.A. The Contractile Phenotype of Dermal Fetal Fibroblasts in Scarless Wound Healing. Curr Pathobiol Rep 5, 271–277 (2017). https://doi.org/10.1007/s40139-017-0149-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-017-0149-3