Abstract
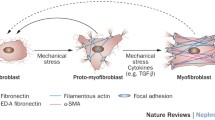
Kidney fibrosis continues to be a topic of enormous biomedical importance as the histologic manifestation of chronic kidney disease regardless of etiology. An active debate surrounds the fundamental issue of identity and ontogeny of the cells responsible for producing renal fibrosis, beginning with the hypothesis that epithelial cells undergo a phenotypic transition to a mesenchymal state (epithelial–mesenchymal transition) to populate the interstitium as myofibroblasts. The subsequent publication of a large body of experimental evidence supports the notion of phenotypic alteration on the part of renal tubular epithelial cells, but their contribution to the interstitial myofibroblast population has not been reproducible. Rather, the emerging concept of a partial epithelial–mesenchymal transition is gaining traction, in which epithelial cells remain localized to the tubule while expressing mesenchymal markers and contributing to fibrosis through signaling. Phenotypic transitions have also been studied in endothelial cells, pericytes, and bone marrow-derived cells. The relative importance of each of these cell types’ contributions to renal fibrosis is an ongoing question. As the fibrotic kidney shows histopathologic alteration in most of its compartments, a likely scenario invokes a complex interaction between tubules, interstitium, and vessels, with gene expression changes and some degree of phenotypic transition across the spectrum of cell types.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Meran S, Steadman R (2011) Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol 92(3):158–167
Hay ED (1968) Organization and fine structure of epithelium and mesenchyme in the developing chick embryo
Davies JAJ (1996) Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat 156(3):187–201
Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG (1995) Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130(2):393–405
Yang J, Liu Y (2001) Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 159(4):1465–1475
Ng Y-Y et al (1998) Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int 54(3):864–876
Rastaldi MP et al (2002) Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int 62(1):137–146
•• Grande MT et al (2015) Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21(9):989–997. This paper provides experimental evidence for partial EMT and shows the importance of the Snail gene in fibrosis development. Epithelial cells remain within tubules while contributing to fibrosis through TGFβ signaling in a process requiring epithelial reactivation of Snail
Jinde K et al (2001) Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis 38(4):761–769
Kaneto H, Morrissey J, Klahr S (1993) Increased expression of TGF-[beta]1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int 44(2):313–321
Böttinger EP, Bitzer M (2002) TGF-ß signaling in renal disease. J Am Soc Nephrol 13(10):2600–2610
Okada H et al (1997) Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol 273(4):F563–F574
Strutz F et al (2002) Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int 61(5):1714–1728
Sato M et al (2003) Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Investig 112(10):1486–1494
Zavadil J et al (2004) Integration of TGF-β/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 23(5):1155–1165
Bielesz B et al (2010) Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Investig 120(11):4040–4054
Boutet A et al (2006) Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25(23):5603–5613
Kida Y et al (2007) Twist relates to tubular epithelial-mesenchymal transition and interstitial fibrogenesis in the obstructed kidney. J Histochem Cytochem 55(7):661–673
Oldfield MD et al (2001) Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Investig 108(12):1853–1863
Higgins DF et al (2007) Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Investig 117(12):3810–3820
He W et al (2009) Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20(4):765–776
Iwano M et al (2002) Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Investig 110(3):341–350
Kriz W, Kaissling B, Le Hir M (2011) Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Investig 121(2):468–474
Galichon P, Finianos S, Hertig A (2013) EMT–MET in renal disease: Should we curb our enthusiasm? Cancer Lett 341(1):24–29
Inoue T et al (2005) Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int 67(6):2488–2493
Humphreys BD et al (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176(1):85–97
Koesters R et al (2010) Tubular overexpression of transforming growth factor-β1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol 177(2):632–643
Bonifer CC (1996) Prerequisites for tissue specific and position independent expression of a gene locus in transgenic mice. J Mol Med 74(11):663–671
•• Lovisa S et al (2015) Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21(9):998–1009. This paper characterizes the partial EMT phenotype in terms of loss of normal tubular epithelial function and regenerative ability, with decreased expression of transporters and cell cycle arrest. These phenotypes as well as fibrosis are dependent on the EMT regulators Twist and Snail
Markwald RR, Fitzharris TP, Smith WN (1975) Structural analysis of endocardial cytodifferentiation. Dev Biol 42(1):160–80
Ma L et al (2005) Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132(24):5601–11
McCulley DJ et al (2008) BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn 237(11):3200–9
Nomura-Kitabayashi A et al (2009) Endoglin is dispensable for angiogenesis, but required for endocardial cushion formation in the midgestation mouse embryo. Dev Biol 335(1):66–77
Wang J et al (2005) Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol 286(1):299–310
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–96
He J et al (2013) Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin Exp Nephrol 17(4):488–97
Medici D, Kalluri R (2012) Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin Cancer Biol 22(5–6):379–84
James D, Rafii S (2014) Maladapted endothelial cells flip the mesenchymal switch. Sci Transl Med 6(227):227fs12
Zeisberg EM et al (2008) Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19(12):2282–7
Li J, Qu X, Bertram JF (2009) Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol 175(4):1380–8
Li J et al (2010) Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes 59(10):2612–24
Shi S et al (2015) Interactions of DPP-4 and integrin [beta]1 influences endothelial-to-mesenchymal transition. Kidney Int 88(3):479–489
Basile DP et al (2011) Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300(3):F721–33
Curci C et al (2014) Endothelial-to-mesenchymal transition and renal fibrosis in ischaemia/reperfusion injury are mediated by complement anaphylatoxins and Akt pathway. Nephrol Dial Transpl 29(4):799–808
• LeBleu VS et al (2013) Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19(8):1047–1053. Using multiple mouse models in a fate-mapping approach to the question of myofibroblast origin, this paper argues the largest contribution to myofibroblasts is local fibroblast proliferation, followed by marrow derived cells. Only a very small minority of myofibroblasts derive from epithelium and endothelium
Ren SS (2013) Pericytes in kidney fibrosis. Curr Opin Nephrol Hypertens 22(4):471–480
Lin S-L et al (2008) Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173(6):1617–1627
Rock JR et al (2011) Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci 108(52):E1475–E1483
Schrimpf C et al (2012) Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23(5):868–883
Broekema M et al (2007) Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen i after ischemia/reperfusion in rats. J Am Soc Nephrol 18(1):165–175
Wada T et al (2011) Involvement of bone-marrow-derived cells in kidney fibrosis. Clin Exp Nephrol 15(1):8–13
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7(12):684–696
Lim J, Thiery JP (2012) Epithelial-mesenchymal transitions: insights from development. Development 139(19):3471–3486
Mani SA et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Matrix Pathobiology.
Rights and permissions
About this article
Cite this article
Ledo, N., Susztak, K. & Palmer, M.B. Cell Phenotype Transitions in Renal Fibrosis. Curr Pathobiol Rep 4, 19–25 (2016). https://doi.org/10.1007/s40139-016-0098-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-016-0098-2