Abstract
Introduction
The study aims to demonstrate and estimate the prevalence of clinical corneal ectasia and keratoconus (KC) in patients with relatively low keratometry (low-K KC).
Methods
In a retrospective, analytical, and non-interventionist study, one eye was randomly selected from 1054 patients from the original Tomographic Biomechanical Index (TBIv1) study and the external validation (from Rio de Janeiro, Brazil, and Milan, Italy clinics). Patients were stratified into three groups. Group 1 included 736 normal patients, and groups 2 and 3 included 318 patients with clinical KC in both eyes, divided into low-K KC (90 patients) and high-K KC (228 patients), respectively. All patients underwent a comprehensive ophthalmological evaluation along with Pentacam and Corvis ST (Oculus, Wetzlar, Germany) examinations. Cases with maximum mean zone 3 mm keratometry (Kmax zone mean 3 mm) lower than 47.6 diopters (D) were considered as low-keratometry keratoconus, and cases with Kmax zone mean 3 mm higher than 47.6 D were regarded as high-keratometry keratoconus.
Results
Ninety (28.30%) of the 318 KC group presented ectasia with low-keratometric values (low-Kmax). The average age in the normal group was 39.28 years (range 6.99–90.12), in the low-Kmax KC group it was 37.49 (range 13.35–78.45), and in the high-Kmax KC group it was 34.22 years (range 12.7–80.34). Mean and SD values and median (range), respectively, of some corneal tomographic and biomechanical parameters evaluated from the low-Kmax KC group were as follows: Belin-Ambrósio enhanced ectasia display (BAD-D) 3.79 ± 1.62 and 3.66 (0.83–9.73); Pentacam random forest index (PRFI) 0.78 ± 0.25 and 0.91 (0.05–1); corneal biomechanical index (CBI) 0.58 ± 0.43 and 0.75 (0–1); TBI 0.93 ± 0.17 and 1 (0.35–1); and stiffness parameter at A1 (SP-A1) 86.16 ± 19.62 and 86.05 (42.94–141.66).
Conclusion
Relatively low keratometry, with a Kmax lower than 47.6 D, can occur in up to 28.30% of clinical keratoconus. These cases have a less severe presentation of the disease. Future studies involving larger populations and prospective designs are necessary to confirm the prevalence of keratoconus with low keratometry and define prognostic factors in such cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Corneal topography has historically been considered a required examination in the screening procedure of refractive surgery candidates. However, it can only analyze the anterior curvature. |
Detection of mild and subclinical forms of ectatic corneal diseases has gained significance because these cases have a high risk of developing iatrogenic progressive ectasia after corneal laser vision correction and frequently go unnoticed when only the front surface of the cornea is examined. |
Beyond corneal topography, multimodal corneal shape analysis and biomechanical assessment are expected to enhance the accuracy of distinguishing between keratoconus, forme fruste keratoconus, and normal patients. |
Low central keratometry does not exclude the patient from having a steep cornea. Clinical corneal ectasia and keratoconus can be associated with relatively low keratometry. |
Introduction
Ectatic corneal disease includes a group of disorders characterized by progressive thinning and succeeding protruding of the corneal structure [1]. While different phenotypes have been documented, keratoconus (KC) is the most common clinical condition. Described by a bilateral noninflammatory ectatic disease characterized by progressive corneal thinning and protrusion [2], its prevalence varies worldwide [3]. Studies show that in the Netherlands, the prevalence is 1 in 345 [4]. It reaches 1.2% of young Australians [5, 6] and up to 4.7% in Arabs [6]. There is a global consensus that KC is a bilateral disease. Still, there is also an agreement that ectasia may occur (in one or both eyes of a patient) as a result of a mechanical process [7]. Currently, the two-hit hypothesis is that environmental factors, such as eye rubbing, atopy, and genetic predisposition, are required to trigger the development and progression of ectatic corneal disease [8].
Placido disc-based corneal topography is sensitive to detect mild changes on the anterior corneal surface with relatively normal distance-corrected vision acuity (DCVA) and biomicroscopy, albeit with a high false positive rate. In the past, Rabinowitz and McDonnell introduced diagnostic criteria for KC based on two indices obtained from corneal topography: a central K value greater than 47.20 diopters (D) and inferior-superior asymmetry (I-S value) greater than 1.4 [9]. Although these criteria sometimes do not provide the most specific results, such data have proved sensitive to identifying mild anterior corneal ectatic patterns. This effectiveness extends even in patients with relatively normal distance spectacle-corrected visual acuity and who show no significant findings in slit lamp examinations [10, 11].
The historical reliance on corneal topography as an essential part of the screening for potential refractive surgery candidates is well founded, given its ability to detect corneal irregularities [12]. Nonetheless, the limitations of focusing exclusively on the cornea’s anterior curvature have become apparent, especially when individuals develop postsurgical keratectasia after refractive surgery despite having a relatively normal anterior shape [13,14,15]. Additionally, there have been instances where patients with abnormal preoperative topographic findings underwent laser vision correction (LVC) based on advanced corneal imaging and achieved stable postoperative results [16]. Therefore, there is a need for a complete characterization of the cornea to improve screening for ectasia susceptibility of refractive candidates.
Identifying early-stage and subclinical ectatic corneal conditions has become increasingly important, as such cases are particularly vulnerable to post-LVC iatrogenic ectasia. The current concept for assessing ectasia risk before refractive surgery is to consider data beyond (not over) the corneal anterior surface with a multimodal approach to characterize ectasia susceptibility [17, 18].
Multimodal corneal imaging involves various technologies in addition to Placido disc corneal topography. These include three-dimensional (3-D) Scheimpflug tomography, which provides measurements from anterior and posterior corneal surfaces and a comprehensive pachymetric map through a 3-D reconstruction of the cornea from limbus to limbus, layered or segmental tomography with Bowman’s and epithelial thickness mapping via OCT (optical coherence tomography), digital very high-frequency ultrasound (VHF-US), and ocular wavefront analysis [3, 19].
Beyond analyzing the shape of the cornea, clinical biomechanical assessments are anticipated to improve precision in detecting early stages of ectatic corneal diseases [20]. They are relevant for diagnosing, staging, and providing prognostic information about the disease [3, 21,22,23]. Studies have verified the capacity of the biomechanical analysis with the Corvis-ST (Oculus, Wetzlar, Germany) to detect mild, forme fruste keratoconus (FFKC) or subclinical KC in eyes with “innocent” and relatively normal anterior topographic maps from patients with contralateral clinical KC [24, 25]. The integration of tomographic and biomechanical data has established the potential to increase the accuracy of detecting ectatic disease and identifying susceptibility to developing this complication after LVC [19, 26, 27].
The study aims to demonstrate clinical corneal ectasia and KC cases in patients with relatively low keratometry (low-K KC) and estimate such prevalence.
Methods
This retrospective, analytical, and non-interventionist study comprised one eye randomly selected from 1054 patients from the original TBIv1 study [19] and the external validation [28] (from the clinics in Rio de Janeiro, Brazil, and Milan, Italy). These patients were divided into three groups: 736 normal and 318 patients with clinical KC in both eyes, divided into 90 low-K KC and 228 high-K KC.
All patients underwent a comprehensive ophthalmological evaluation along with the Pentacam and Corvis ST (Oculus, Wetzlar, Germany) examinations. The main parameters evaluated were corneal biomechanical index (CBI), Pentacam random forest index (PRFI), Belin-Ambrósio enhanced ectasia display (BAD-D), tomographic biomechanical index (TBI), stiffness parameter at A1 (SP-A1), front surface elevation at the apex point using the 8 mm best-fit sphere (Ele FBFS 8 mm apex), front surface elevation at the thinnest point using the 8 mm best-fit sphere (Ele FBFS 8 mm thinnest), front surface elevation at the maximum 4 mm zone using the 8 mm best-fit sphere (Ele FBFS 8 mm max 4 mm zone), back surface elevation at the apex point using the 8 mm best-fit sphere (Ele BBFS 8 mm apex), back surface elevation at the thinnest point using the 8 mm best-fit sphere (Ele BBFS 8 mm thinnest), back surface elevation at the maximum 4 mm zone using the 8 mm best-fit sphere (Ele BBFS 8 mm max 4 mm zone), front surface asphericity at 30 degrees (Asph Q front 30°), and back surface asphericity at 30 degrees (Asph Q back 30°).
Patients with a maximum zone mean of 3 mm keratometry (Kmax zone mean 3 mm) measured in the Pentacam lower than 47.6 D were considered low-keratometry KC, and cases with Kmax zone mean 3 mm higher than 47.6 D were considered as high-keratometry KC. The criterion is based on Rabinowitz’s studies, corroborated by the literature and our clinical experience [9].
Data were collected, revised, coded, and entered into the R software. The quantitative data were presented as mean, standard deviations (SD), and ranges when parametric, and the qualitative variables were presented as numbers and percentages. The groups were compared using the non-parametric Kruskal–Wallis test and the post hoc Dunn’s test to compare each pair of groups. The confidence interval was 95%, and the accepted error margin was 5%. So, the p value was considered significant if less than 0.05.
The ethics committee approved the proposed investigation at the Federal University of São Paulo/UNIFESP/SP 2020 (# 4.050.934). Patient consent was not needed as this is a retrospective study using a database and no identifiable information was used.
Results
Ninety (28.30%) of the 318 cases in the group with KC had ectasia with low Kmax, and the mean with standard deviation (SD) and median (range), respectively, Kmax in the low-KC group were 46.15 ± 1.02 and 46.28 (43.09–47.58). Considering the remaining patients from the group with KC, 228 (71.7%) had ectasia with high Kmax, and the mean Kmax was 52.98 ± 4.55 and 51.93 (47.81–76.78).
The mean ± SD and median (range) age in the normal group were 39.28 ± 16.97 and 34.77 (6.99–90.12) years; those in the low-Kmax KC group were 37.49 ± 14.69 and 33.89 (13.35–78.45) years; and those in the high-Kmax KC group were 34.22 ± 12.8 and 32.37 (range 12.7–80.34) years.
The mean ± SD and median (range) pachymetry in the normal group were 544 ± 32.51 and 543.2 (454–680); those in the low-Kmax KC group were 500 ± 35.27 and 500.04 (401–597); and those in the high-Kmax KC group were 474 ± 38.67 and 475.91 (323–561) (Table 1).
Mean and SD values and median (range), respectively, of tomographic and biomechanical parameters in the normal group were BAD-D 0.78 ± 0.61 and 0.8 (− 1.41 to 4.81); PRFI 0.07 ± 0.08 and 0.04 (0–0.74); CBI 0.05 ± 0.13 and 0 (0–0.99); TBI 0.08 ± 0.12 and 0.02 (0–0.59), and SP-A1 110.66 ± 19.96 and 109.56 (31.05–218.9). In the low-Kmax KC group these were BAD-D 3.79 ± 1.62 and 3.66 (0.83–9.73); PRFI 0.78 ± 0.25 and 0.91 (0.05–1); CBI 0.58 ± 0.43 and 0.75 (0–1); TBI 0.93 ± 0.17 and 1 (0.35–1); and SP-A1 86.16 ± 19.62 and 86.05 (42.94–141.66). In the high-Kmax KC group these were BAD-D 8.5 ± 3.73 and 7.86 (1.55–26.81); PRFI 0.97 ± 0.1 and 0.99 (0.05–1); CBI 0.94 ± 0.19 and 1 (0–1); TBI 0.99 ± 0.05 and 1 (0.44–1); and SP-A1 67.88 ± 19.37 and 68.48 (22.79 to121.76) (Table 1).
The front and back elevations from the best-fit sphere, the front and back surface asphericity at 30°, and other tomographic and biomechanics parameters in each group are also described in Table 1.
Considering the most cited tomographic and biomechanics parameters, 40% of the patients in the low-K KC group had CBI lower than 0.5 and 36.6% lower than 0.3, 17.7% had PRFI lower than 0.5 and 2.2% lower than 0.15, and 4.4% had TBI lower than 0.5. No patient had TBI lower than 0.3.
Table 1 compares the main tomographic and biomechanical parameters between the normal group vs. high-K KC, normal vs. low-K KC, and low-K KC vs. high-K KC groups; statistically significant differences were found in the Dunn’s test for Kruskal–Wallis. There is also a statistically significant difference between the normal group vs. low-K KC and low-K KC vs. high-K KC groups; the low-K KC group presents tomographic changes to the normal and high-K KC groups. Only the comparisons of K1 and Asph Q back 30° between normals vs. low-K KC, and for PRFI, and TBI between low-K KC vs. high-K KC groups were not statistically significant.
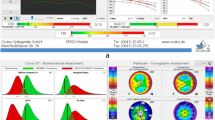
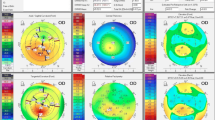
We show a case of a 65-year-old man with DCVA of 20/20: (− 3.00/− 0.50 × 75) in the right eye (OD) and 20/20 (− 2.50/− 0.50 × 166) in the left eye (OS). He presents Placido topometric changes typical of KC in both eyes with inferior steepening and relatively low keratometry (Kmax of 43.2 D in the OD and 44.5 D in the OS) (Fig. 1), with changes in the tomographic (Fig. 2) and biomechanical parameters (Fig. 3) [17].
The Pentacam topometric shows the topography of a low keratometry keratoconus (KC) with a keratometry (K) max of 43.2, keratoconus percentage index (KISA) of 24.543, and ISV of 29 in the right eye (OD) (A), and Kmax of 43.5, KISA of 5.044 and index of surface variance (ISV) of 37 in the left eye (OS) (B)
Corvis ST tomographic-biomechanical display of the patient with low keratometry KC showing a tomographic biomechanical index (TBI) of 1.0 in the OD (A) and 0.96 in the OS (B), a corneal biomechanical index (CBI) of 0.37 in the OD (A) and 0.25 in the OS (B), and a Pentacam random forest index (PRFI) of 0.92 in the OD (A) and 0.79 in the OS (B). OD = right eye; OS = left eye
Discussion
Low central keratometry does not exclude the patient from having a steep cornea. Several studies in the past have already demonstrated, through corneal topography, the presence of KC with low keratometry.
Rabinowitz and McDonnell’s 1989 study, also based on previous studies on the topic, established that central corneal power was steeper in KC eyes when compared to normal eyes; the mean central power found in KC eyes was 52.13 (range 43.10–60.20), and four eyes had a central power of less than 46.7 D (less than two standard deviations above the normal) [9]. A former study reported no absolute cutoff value for the steepness of the central cornea when making a KC diagnosis because there were patients with steep corneas and high astigmatic errors who did not have KC and patients with definite KC in the 39 D range [1].
The literature reported a case of a patient with no evidence of KC in a biomicroscopic examination but a subtle videokeratoscopic diagnosis of the disease, with Kmax with 45.5 D at the cone apex [29]. Another report observed a patient who presented with suspected KC in one eye with Kmax of 44.5 D and clinical KC in the other eye, and over 2 years, the cone apex power increased to 51.0 D [30].
Beyond corneal topography, which has the disadvantage of evaluating only the anterior curvature of the cornea, multimodal corneal shape analysis with other technology and clinical biomechanical assessment is expected to enhance the accuracy of distinguishing between KC, FFKC, and normal patients [15]. The global consensus is that although KC may present with a high degree of asymmetry, the disease is typically bilateral [7].
Comprehending the biomechanical properties of the cornea is crucial for identifying subclinical KC and the progression of ectasia. However, changes in corneal topography remain inadequate and fail to provide definitive evidence [26]. The combined use of tomographic and biomechanical data has shown promise in enhancing the accuracy of detecting ectatic disorders [19, 26, 27, 31]. The optimized TBI study (TBIV2) specified some diagnostic criteria; according to Rabinowitz, an IS value asymmetry within 6 mm, and a classical cutoff of 1.6 results in a specificity of 99.6% and a sensitivity of 5.1% for VAE-NT cases, and 84.5% for clinical ectasia [2, 32]. Reducing the cutoff to 1.45 D decreased the specificity to 99.3% while increasing sensitivity to 86.6% for clinical ectasia and 6.9% for VAE-NT cases. Using a Kmax cutoff of 47.6 D achieves a specificity of 97.7% and a sensitivity of 82.1% for clinical ectasia but only 4.9% for VAE-NT. Notably, 8% of patients with clinical ectasia exhibited a Kmax below 46 D and 3.3% below 45 D. Furthermore, an ART-max [33] cutoff of 329 μm results in a specificity of 98% and a sensitivity of 93.7% for clinical ectasia but just 34.7% for VAE-NT. Adjusting the ART-max cutoff to 387 μm reduces specificity to 83.6%, with a sensitivity of 65.7% for VAE-NT.
Effective screening for refractive surgery involves more than just detecting mild cases of ectasia; it is essential to identify individuals at high susceptibility or predisposed to biomechanical failure and ectasia following LVC [18, 34]. Retrospective studies on patients who developed ectasia after LASIK (laser-assisted in situ keratomileusis) have shown that corneal tomographic parameters effectively detect susceptibility to this condition [33, 35, 36]. Furthermore, there is a recognized need to expand beyond assessing corneal shape and include biomechanical considerations in evaluating the risk of ectasia [37, 38].
The biomechanical analyses by the Corvis ST utilize dynamic testing to investigate corneal deformation parameters by examining how the cornea responds to stress. Artificial intelligence (AI) algorithms have verified that organizing these deformation parameters enhances the ability to accurately differentiate between healthy eyes and those with KC, even in mild stages [39]. Additionally, the waveform analysis by the Corvis ST of deformation and deflection amplitude signals has proven exceptionally effective in distinguishing between normal, suspect, and KC eyes [40].
Describing the genetics of KC is intensely challenging. The development of KC has been linked to multiple genes [41]. Research identifying 17 different genomic loci in patients with KC illustrates the genetic diversity of the condition [42]. This is further supported by findings showing KC can follow autosomal dominant and recessive patterns [43]. Shortly, molecular biology could become crucial for diagnosing, classifying, and allowing personalized treatments.
Patients and their families should understand that KC surgery is intended for therapeutic purposes, not refractive surgery [44]. The primary goal of such surgical interventions is to restore vision. Deciding whether to perform a procedure and determining the most suitable method for each patient requires personalized consideration. Additionally, patient compliance must include advice to avoid eye rubbing, treat ocular allergies, and optimize the ocular surface [45].
This study has limitations. As it is a retrospective study, we could not correlate our data with anamnesis or visual acuity without and with correction and the wavefront examination. Furthermore, it would be interesting to correlate with studies involving these patients’ genetics and molecular biology.
Conclusion
Our study shows clinical corneal ectasia and KC can be associated with relatively low keratometry, with Kmax zone means of 3 mm lower than 47.6 D. Future studies are needed to evaluate the prevalence in larger populations. Therefore, we can better diagnose, classify, assess prognosis, and offer the best treatment for those patients.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293–322.
Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. https://doi.org/10.1016/s0039-6257(97)00119-7.
Salomão M, Hoffling-Lima AL, Lopes B, et al. Recent developments in keratoconus diagnosis. Expert Rev Ophthalmol. 2018;13(6):329–41.
Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RP. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017;175:169–72. https://doi.org/10.1016/j.ajo.2016.12.015.
Chan E, Chong EW, Lingham G, et al. Prevalence of keratoconus based on Scheimpflug imaging: the Raine study. Ophthalmology. 2021;128(4):515–21.
Torres Netto EA, Al-Otaibi WM, Hafezi NL, et al. Prevalence of keratoconus in paediatric patients in Riyadh Saudi Arabia. Br J Ophthalmol. 2018;102(10):1436–41. https://doi.org/10.1136/bjophthalmol-2017-311391.
Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–69. https://doi.org/10.1097/ICO.0000000000000408.
McGhee CN, Kim BZ, Wilson PJ. Contemporary treatment paradigms in keratoconus. Cornea. 2015;34(Suppl 10):S16-23. https://doi.org/10.1097/ICO.0000000000000504.
Rabinowitz YS, McDonnell PJ. Computer-assisted corneal topography in keratoconus. Refract Corneal Surg. 1989;5(6):400–8.
Maeda N, Klyce SD, Smolek MK, Thompson HW. Automated keratoconus screening with corneal topography analysis. Invest Ophthalmol Vis Sci. 1994;35(6):2749–57.
Maguire LJ, Bourne WM. Corneal topography of early keratoconus. Am J Ophthalmol. 1989;108(2):107–12.
Ambrósio R Jr, Klyce SD, Wilson SE. Corneal topographic and pachymetric screening of keratorefractive patients. J Refract Surg. 2003;19(1):24–9.
Klein SR, Epstein RJ, Randleman JB, Stulting RD. Corneal ectasia after laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea. 2006;25(4):388–403.
Malecaze F, Coullet J, Calvas P, Fournié P, Arné J-L, Brodaty C. Corneal ectasia after photorefractive keratectomy for low myopia. Ophthalmology. 2006;113(5):742–6.
Ambrósio R Jr, Dawson DG, Salomao M, Guerra FP, Caiado AL, Belin MW. Corneal ectasia after LASIK despite low preoperative risk: tomographic and biomechanical findings in the unoperated, stable, fellow eye. J Refract Surg. 2010;26(11):906–11. https://doi.org/10.3928/1081597X-20100428-02.
Reinstein DZ, Archer TJ, Gobbe M. Stability of LASIK in topographically suspect keratoconus confirmed non-keratoconic by Artemis VHF digital ultrasound epithelial thickness mapping: 1-year follow-up. J Refract Surg. 2009;25(7):569–77.
Ambrósio R Jr, Salomão MQ, Barros L, et al. Multimodal diagnostics for keratoconus and ectatic corneal diseases: a paradigm shift. Eye Vis. 2023;10(1):45.
Ambrósio R Jr, Randleman JB. Screening for ectasia risk: what are we screening for and how should we screen for it? J Refract Surg. 2013;29(4):230–2. https://doi.org/10.3928/1081597X-20130318-01.
Ambrósio R Jr, Lopes BT, Faria-Correia F, et al. Integration of Scheimpflug-based corneal tomography and biomechanical assessments for enhancing ectasia detection. J Refract Surg. 2017;33(7):434–43. https://doi.org/10.3928/1081597x-20170426-02.
Ambrósio R Jr, Nogueira LP, Caldas DL, et al. Evaluation of corneal shape and biomechanics before LASIK. Int Ophthalmol Clin. 2011;51(2):11–38. https://doi.org/10.1097/IIO.0b013e31820f1d2d.
Sedaghat MR, Ostadi-Moghadam H, Jabbarvand M, Askarizadeh F, Momeni-Moghaddam H, Narooie-Noori F. Corneal hysteresis and corneal resistance factor in pellucid marginal degeneration. J Curr Ophthalmol. 2018;30(1):42–7. https://doi.org/10.1016/j.joco.2017.08.002.
Ambrósio R Jr, Correia FF, Lopes B, et al. Corneal biomechanics in ectatic diseases: refractive surgery implications. Open Ophthalmol J. 2017;11:176–93. https://doi.org/10.2174/1874364101711010176.
Esporcatte LPG, Salomao MQ, Lopes BT, et al. Biomechanical diagnostics of the cornea. Eye Vis (Lond). 2020;7:9. https://doi.org/10.1186/s40662-020-0174-x.
Ambrósio R Jr, Valbon BF, Faria-Correia F, Ramos I, Luz A. Scheimpflug imaging for laser refractive surgery. Curr Opin Ophthalmol. 2013;24(4):310–20. https://doi.org/10.1097/ICU.0b013e3283622a94.
Smadja D, Touboul D, Cohen A, et al. Detection of subclinical keratoconus using an automated decision tree classification. Am J Ophthalmol. 2013;156(2):237–246.e1. https://doi.org/10.1016/j.ajo.2013.03.034.
Bao F, Geraghty B, Wang Q, Elsheikh A. Consideration of corneal biomechanics in the diagnosis and management of keratoconus: is it important? Eye Vis. 2016;3(1):18.
Ferreira-Mendes J, Lopes BT, Faria-Correia F, Salomão MQ, Rodrigues-Barros S, Ambrósio R Jr. Enhanced ectasia detection using corneal tomography and biomechanics. Am J Ophthalmol. 2019;197:7–16.
Salomao MQ, Hofling-Lima AL, Gomes Esporcatte LP, et al. The role of corneal biomechanics for the evaluation of ectasia patients. Int J Environ Res Public Health. 2020;17(6):2113. https://doi.org/10.3390/ijerph17062113.
Harrison DA, Maguire LJ. Biomicroscopic evidence of keratoconus with an apex power of 45.5 diopters by videokeratoscopy. Am J Ophthalmol. 1995;119(3):366–7. https://doi.org/10.1016/s0002-9394(14)71183-3.
Maguire LJ, Lowry JC. Identifying progression of subclinical keratoconus by serial topography analysis. Am J Ophthalmol. 1991;112(1):41–5. https://doi.org/10.1016/s0002-9394(14)76210-5.
Vinciguerra R, Rehman S, Vallabh NA, et al. Corneal biomechanics and biomechanically corrected intraocular pressure in primary open-angle glaucoma, ocular hypertension and controls. Br J Ophthalmol. 2019. https://doi.org/10.1136/bjophthalmol-2018-313493.
Ambrósio R Jr, Machado AP, Leao E, et al. Optimized artificial intelligence for enhanced ectasia detection using scheimpflug-based corneal tomography and biomechanical data. Am J Ophthalmol. 2023;251:126–42. https://doi.org/10.1016/j.ajo.2022.12.016.
Ambrósio R, Caiado ALC, Guerra FP, et al. Novel pachymetric parameters based on corneal tomography for diagnosing keratoconus. J Refract Surg. 2011;27(10):753–8.
Belin MW, Ambrósio R Jr. Corneal ectasia risk score: statistical validity and clinical relevance. J Refract Surg. 2010;26(4):238–40. https://doi.org/10.3928/1081597X-20100318-01.
Ambrósio R Jr, Belin M. Enhanced screening for ectasia risk prior to laser laser vision correction. Int J Keratoconus Ectatic Corneal Dis. 2017;6(1):23–33. https://doi.org/10.5005/jp-journals-10025-1139.
Ambrósio Junior R, Caldas DL, Silva RSd, Pimentel LN, Valbon BDF. Impacto da análise do “wavefront” na refratometria de pacientes com ceratocone. Rev Bras Oftalmol. 2010;69:294–300.
Belin MW, Khachikian SS, Salomão M, Ambrósio R Jr. Keratoconus and ectasia detection based on elevation dat with the Oculus Pentacam®. In: Kiliç A, Roberts CJ, editors. Corneal topography from theory to practice. Netherlands: Kugler; 2013. p. 167.
Luz A, Faria-Correia F, Salomão MQ, Lopes BT, Ambrósio R Jr. Corneal biomechanics: where are we? J Curr Ophthalmol. 2016;28(3):97.
Salomao MQ, Hofling-Lima AL, Faria-Correia F, et al. Dynamic corneal deformation response and integrated corneal tomography. Indian J Ophthalmol. 2018;66(3):373–82. https://doi.org/10.4103/ijo.IJO_831_17.
Francis M, Pahuja N, Shroff R, et al. Waveform analysis of deformation amplitude and deflection amplitude in normal, suspect, and keratoconic eyes. J Cataract Refract Surg. 2017;43(10):1271–80. https://doi.org/10.1016/j.jcrs.2017.10.012.
Abu-Amero KK, Kalantan H, Al-Muammar AM. Analysis of the VSX1 gene in keratoconus patients from Saudi Arabia. Mol Vis. 2011;17:667–72 ((In eng)).
Nowak DM, Gajecka M. The genetics of keratoconus. Middle East Afr J Ophthalmol. 2011;18(1):2–6. https://doi.org/10.4103/0974-9233.75876.
Stabuc-Silih M, Strazisar M, Ravnik-Glavac M, Hawlina M, Glavac D. Genetics and clinical characteristics of keratoconus. Acta Dermatovenerol Alp Pannon Adriat. 2010;19(2):3–10.
Ambrósio R Jr. Cirurgia refrativa terapêutica: por que diferenciar? Rev Bras Oftalmol. 2013;72:85–6.
Esporcatte LPG, Salomão MQ, Neto ABdC, Machado AP, Lopes BT, Ambrósio R Jr. Enhanced diagnostics for corneal ectatic diseases: the whats, the whys, and the hows. Diagnostics. 2022;12(12):3027.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors (Louise Esporcatte, Marcella Salomão, Bernardo Lopes, Nelson Sena Jr., Aydano Machado, Paolo Vinciguerra, Riccardo Vinciguerra, and Renato Ambrósio Jr.) contributed to the study’s conception and design. Louise Pellegrino Gomes Esporcatte wrote the first draft of the manuscript, and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Louise Esporcatte, Marcella Salomão, Bernardo Lopes, Nelson Sena Jr., Aydano Machado, Paolo Vinciguerra, and Riccardo Vinciguerra declare that they have no competing interests. Dr. Renato Ambrósio Jr. is an Editorial Board member of Ophthalmology and Therapy. Renato Ambrósio did not select peer reviewers for the manuscript or any subsequent editorial decisions.
Ethical Approval
The ethics committee approved the proposed investigation at the Federal University of São Paulo/UNIFESP/SP 2020 (# 4.050.934). Patient consent was not needed as this is a retrospective study using a database and no identifiable information was used.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Esporcatte, L.P.G., Salomão, M.Q., Lopes, B.T. et al. Keratoconus and Corneal Ectasia with Relatively Low Keratometry. Ophthalmol Ther 13, 2023–2035 (2024). https://doi.org/10.1007/s40123-024-00964-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00964-5