Abstract
Preretinal deposits (PDs) are a rare condition among fundus diseases. We found that preretinal deposits have some features in common that can provide clinical information. This review affords an overview of PDs in different but related ocular diseases and events, and summarizes the clinical features and possible origin of PDs in related conditions, providing diagnostic clues for ophthalmologists when facing PDs. A literature search was performed using three major electronic databases (PubMed, EMBASE, and Google Scholar) to identify potentially relevant articles published on or before June 4, 2022. Most of the cases in the enrolled articles had optical coherence tomography (OCT) images to confirm the preretinal location of the deposits. Thirty-two publications reported PD-related conditions, including ocular toxoplasmosis (OT), syphilitic uveitis, vitreoretinal lymphoma, human T-cell lymphotropic virus type 1 (HTLV-I) associated uveitis or HTLV-I carriers, acute retinal necrosis, endogenous fungal endophthalmitis, idiopathic uveitis, and exogenous materials. Based on our review, OT is the most frequent infectious disease to exhibit PDs, and silicone oil tamponade is the most common exogenous cause of preretinal deposits. PDs in inflammatory diseases are highly suggestive of active infectious disease and are preferentially accompanied by a retinitis area. However, PDs will largely resolve after etiological treatment in either inflammatory or exogenous conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Preretinal deposits (PDs) are a clinical sign that is often neglected by most ophthalmologists. PDs in different entities may have something in common. PDs occur more often in inflammatory conditions related to infection (i.e., ocular toxoplasmosis, syphilitic uveitis, acute retinal necrosis, and endogenous fungal endophthalmitis) than in non-infectious conditions. |
PDs in inflammatory conditions are likely to have two sources—a retinitis area and the vitreous cavity. |
Sign of PDs is a predictor suggestive of an active state of disease, and probably provides information that can indicate an appropriate treatment. |
PDs have a good prognosis in inflammatory or exogenous conditions, and commonly gradually resolve 1–4 weeks after the etiological treatment was given. |
Introduction
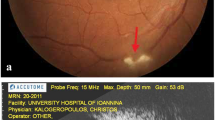
Preretinal deposits (PDs) were first reported by Nakao et al. in a cohort with human T-cell lymphotropic virus type 1 (HTLV-I) associated uveitis (HAU) in 1996. In Nakao’s study, 8 of 55 cases showed gray-white, granular PDs scattered on the retinal veins and/or arteries in the posterior pole. These PDs resolved in a few weeks spontaneously or in response to corticosteroids together with anterior uveal inflammation [1]. Subsequently, PDs were reported in different diseases, including infectious diseases (ocular toxoplasmosis (OT) [2,3,4,5,6,7,8], syphilitic uveitis [9,10,11,12,13], vitreoretinal lymphoma [VRL] [5, 14,15,16,17], HAU or HTLV-I carriers [1, 18], acute retinal necrosis (ARN) [19, 20], and endogenous fungal endophthalmitis [21,22,23,24,25]) and uveitis of uncertain etiology (Fig. 1) [6, 14]. In some iatrogenic conditions, exogenous materials comprising silicone oil (SO) tamponade [26,27,28,29,30], intravitreal antibiotics [31, 32], and the suspicious vitreous remnant of a viscoelastic substance [33] can also occur as PDs.
The pattern and accompanying manifestations of PDs could provide some diagnostic clues to related diseases. The oval PDs visualized in optical coherence tomography (OCT) have been proposed to be a suggestive sign of toxoplasmic etiology in cases of necrotizing retinitis of unknown cause [2]. In addition, herpes zoster virus and syphilis are highly suspected when PDs occur in necrotizing retinitis [10]. In patients with HAU, the PDs adhered to the retinal vessels, suggesting a characteristic sign for HTLV-I associated uveitis [1]. Therefore, PDs have meaningful diagnostic value to some extent.
We found a lot of publications reporting preretinal findings similar to PDs; however, there are no publications summarizing this interesting manifestation. Therefore, a comprehensive literature review of PDs was conducted to provide a better understanding of PDs in fundus diseases.
Literature Search Strategy and Enrollment
The literature review was performed by two investigators (Y.C. and C.C.). The Ethics Committee of Beijing Tongren Hospital affiliated to Capital Medical University waived the need for formal approval of this study given the nature of the study, and only data which had already been published were used. Full consent for the procedures described in Fig. 1 was obtained from the patient. A literature search was performed using three major electronic databases (PubMed, EMBASE, and Google Scholar) to identify potentially relevant articles published on or before June 4, 2022. The following keywords were used for the PubMed search: “((preretinal OR epiretinal)) AND (deposits),” “((preretinal OR epiretinal)) AND (precipitates),” and “((preretinal OR epiretinal)) AND (condensation).” Search strategies for other databases were adapted from the initial PubMed strategy to meet the requirements of each database. All reference lists of the obtained articles were manually screened to identify potentially relevant articles. Given the rarity of the entity, case–control studies, cohort studies, case series, and case reports were considered for inclusion. Letters or correspondence addressing clinical cases were also considered for inclusion. The enrolled articles had to be in English or to have an English abstract. The cases in the enrolled articles had to have OCT images to confirm the preretinal location of the deposits. However, given the rarity of the condition, some reported cases that had no OCT images but did have definite ‘preretinal’ descriptions were also enrolled due to the limitations of the imaging technology in the previous era.
Results
After the exclusion of publications not written in English as well as those without an English abstract and those on vitreous cavity (VC) deposits and keratic precipitates and other unrelated conditions, we found 32 publications (127 cases and 129 eyes) that undoubtedly referred to preretinal deposits; related entities included ocular toxoplasmosis (OT) [2,3,4,5,6,7,8], syphilitic uveitis [9,10,11,12,13], vitreoretinal lymphoma (VRL) [5, 14,15,16,17], virus-related uveitis HTLV-I associated uveitis (HAU) or HTLV-I carriers [1, 18] and acute retinal necrosis (ARN) [19, 20], endogenous fungal endophthalmitis [21,22,23,24,25], uveitis of uncertain etiology [6, 14], exogenous materials comprising silicone oil (SO) tamponade [26,27,28,29,30], intravitreal antibiotics [31, 32], and the suspicious vitreous remnant of viscoelastic substance (two cases in one publication) [33]. Three publications each described two cases of PDs with different causes [5, 6, 14]. Overall, OT was the infectious entity that most commonly presented with PDs, and SO tamponade was the most common exogenous cause of PDs (Table 1).
Preretinal Deposits in Ocular Toxoplasmosis
Ocular toxoplasmosis (OT), caused by the protozoan parasite Toxoplasma gondii, is the leading cause of posterior uveitis in the world, accounting for 20–80% of the cases [34]. The retina is the primary site of the infection, but the choroid, vitreous, and anterior chamber are also involved secondarily by inflammation [35]. Several studies have reported PDs in OT visualized on OCT, especially in toxoplasmic retinochoroiditis (TRC) [2,3,4, 7, 8]. Gass initially described the possible presence of multiple small, granular deposits that might develop along the inner retinal surface in the vicinity of acute toxoplasmic retinitis in his textbook [36]. The preretinal oval deposits visualized in OCT have been proposed to be a suggestive sign of toxoplasmic etiology in the case of necrotizing retinitis of unknown cause, yet herpes zoster virus and syphilis are differential etiologies [2,3,4, 7, 8]. The prevalence of oval PDs in retrospective studies of TRC ranged from 36.0% to 83.7% [4, 7, 8]. The deposits are mostly oval or round in shape and gray in color, and may have a dot-like shape. The deposits distribute along the retinal veins and arteries and/or in the macular region. On OCT, the hyperreflective oval deposits occur on the vitreoretinal interface, adjacent to or far away from the active lesions with diameters of 100–150 μm [2]. The deposits are often seen at presentation; however, they gradually become smaller and fade over time until they completely resolve after treatment with systematic antimicrobial drugs and steroids. Notably, Goldenberg documented that the deposits could enter the inner retinal layers and then resolve completely [3]. The deposits may represent clumps of inflammatory cells and/or parasitic cysts [37]. Apart from PDs, deposits in OT patients can also be found hanging on the thickened posterior hyaloid or suspended in the vitreous cavity. Yonekawa et al. reported a case with TRC that showed diffuse stalagmite-like PDs in a vitrectomized eye, and the authors believe that the inflammatory cells can move more freely in a vitrectomized eye via Brownian-like motion, in contrast to deposits in nonvitrectomized eyes where vitreous debris tends to accumulate inferiorly or at the fovea [5]. Additionally, Ksiaa et al. conducted a study of distinguishing OCT findings in active TRC and found that retinal hyperreflective round PDs, in conjunction with sub-lesional choroidal thickening and sub-lesional retinal pigment epithelium elevation, are more likely to occur in OT patients [8]. Their findings indicate that the presence of deposits, along with other OCT signs, provides some diagnostic clues for OT.
Preretinal Deposits in Syphilitic Uveitis
Acquired syphilis is generally considered a sexually transmitted disease caused by Treponema pallidum [38]. Ocular manifestations happen commonly in the secondary and tertiary stages of syphilis, although ocular involvement can occur in all stages [39]. Treponema can impair all the layers of the eyeball, which explains its various clinical presentations [40]. Syphilis has been considered “the great mimicker” because it lacks pathognomonic signs and often presents with manifestations similar to various other ocular diseases [38,39,40,41]. Until recently, some ophthalmologists reported that PDs, also called precipitates, overlying the areas of retinitis in syphilitic patients with ocular involvement were highly suggestive of ocular syphilis [9,10,11,12,13, 42, 43]. Meyer et al. reported a young man with syphilitic retinitis who showed multiple small, yellow, preretinal precipitates (dots) [42]. Subsequently, Reddy et al. documented a 42-year-old male with ocular syphilis who showed inflammatory precipitates overlying an area of retinitis and used OCT to confirm the preretinal location [9]. Fu et al. described a series of nine eyes from eight patients with syphilitic retinitis who presented with creamy yellowish superficial, multifocal, retinal precipitates overlying an area of retinitis [10]. Most eyes (66.7%) presented a single group of precipitates, while others (33.7%) presented multiple groups of precipitates associated with distinct areas of retinitis. The precipitates completely resolved within 1–2 weeks after antibiotic treatment. Rodrigues et al. investigated the characteristics of yellowish dots in 12 patients and found that retinal precipitates were usually located in the perivascular region. They assumed that the preretinal dots were due to perivasculitis secondary to Treponema infection [12]. The aforementioned findings suggested by these authors indicate that preretinal precipitates or pale yellowish dots in conjunction with an underlying ground-glass retinitis area are highly suggestive of ocular syphilis. As for the nature or composition of the PDs, it remains unknown, and there is no histological evidence to reveal its composition. Yang et al. hypothesized that the deposits originated from Treponema collections and inflammatory cells located preretinally and intraretinally [11], whereas Fu et al. considered that the precipitates were clots of white blood cells [10]. All in all, the round yellowish-white PDs commonly occur on a ground-glass retinitis area in a single cluster or in multiple distributions, regardless of immune status, human immunodeficiency virus positivity, and sexual preference [10], and they can be located around vessels or in the macular area. Notably, some deposits were observed to migrate across the inflamed retina during the evolution of the infection and its treatment [43]. The precipitates, in conjunction with a positive pathogen test and other related ocular conditions (retinitis, retinal vasculitis, and vitritis), probably provide diagnostic value to ophthalmologists. However, in our review of the literature, only two publications presented the OCT appearance of preretinal precipitates, so more investigations using new OCT technology to observe the features of the precipitates should be conducted in the future.
Preretinal Deposits in Vitreoretinal Lymphoma
Vitreoretinal lymphoma (VRL) is a rare ocular malignancy with an incidence of under 3% among intraocular tumors [44]. It is considered to be a subtype of central nervous system (CNS) lymphomas that predominantly affects the vitreous and/or retina, and usually resembles chronic intermediate or posterior uveitis [45, 46]. VRL can present with various nonspecific ocular manifestations, including vitreous cells and multifocal, yellowish-white deposits in or under the retina and beneath retinal pigment epithelium (RPE). Recently, PDs in VRL have been reported as a novel OCT finding at presentation or recurrence [5, 15,16,17]. The prevalence of PDs in retrospective studies of VRL ranged from 10.5% to 26.3% [15,16,17]. Saito et al. reported that PDs, which are described as villous-shaped lesions on the retina, occurred in one patient undergoing pretreatment and three patients experiencing a recurrence (multiple (3–4) episodes) in a cohort of 38 patients with VRL, indicating that PDs occur more often in recurrent cases than during pretreatment [15]. Yang et al. noticed that, among the recurrent cases, 1 of the 15 eyes showed PDs at recurrence, while this eye had no PDs at presentation. Notably, Yonekawa et al. reported a uveitis case showing stalagmite-like PDs, where the patient had a history of intraocular lymphoma that had been in regression after a diagnostic vitrectomy without further treatment [5]. In the light of OCT findings, in addition to a preretinal location, the deposits can be located in the sub-RPE, sub-retinal, and intraretinal spaces. Yang et al. found that intraretinal deposits significantly increase at maximal progression and recurrence in comparison with the initial presentation, suggesting that the development of intraretinal deposits is likely to be a predictor of disease progression or recurrence [16]. Conversely, Saito et al. noticed that subretinal deposits and RPE irregularities occurred more often in recurrent patients [15]. Regarding the composition of these deposits, lymphoma cells are considered to be the major component, and cells from the VC are likely to be the source of the preretinal deposits. Notably, the patients in the aforementioned studies were classified as having large B-cell VRL, so the composition of the PDs in VRL is reasonably assumed to be B-cell lymphoma cells. Although the site of VRL onset and the development pattern of VRL are not clear, some researchers have proposed that the sub-RPE space and the VC are the origin [16, 47, 48]. So, it is reasonable to speculate that the PDs from the VC may migrate through the retina into the subretinal space. The PDs can disappear after vitrectomy or during regression [16, 17]. Since the PDs in VRL are not a pathognomonic sign and exert little impact on the prognosis, many OCT images showing PDs are not included in VRL-related publications, and the PDs are not mentioned in them [49, 50]. Overall, PDs occur in active VRL but seem to provide little diagnostic value currently.
Preretinal Deposits in Virus-Related Uveitis
In our review of the literature, we found PDs documented in both human HAU and ARN [1, 18, 20]. HTLV-I is the first reported retrovirus that is involved in the etiology of human diseases, including hematologic malignancy and neurological diseases [51]. HAU is an inflammatory disease affecting the uvea that is associated with HTLV-I and mainly occurs in middle-aged adults of either sex, with a prevalence of roughly 112.2 per 10,000 HTLV-I carriers [52, 53]. HAU can show acute, granulomatous, or non-granulomatous uveitis, presenting with symptoms such as foggy vision, ocular floaters, blurring of vision, ocular hyperemia, ocular pain, and photophobia, and anterior inflammatory and retinal vasculitis, with unilateral or bilateral involvement [54, 55]. Nakao et al. first described PDs in eight cases with HAU in 1996 [1]. They noticed that the PDs were gray-white in appearance, granular in shape, and scattered on the retinal veins and/or arteries in the posterior pole. The PDs, also called vascular lesions in their study, showed no complications (hemorrhage, vascular sheathing, or leakage points) on fundus fluorescence angiography (FFA). The PDs completely resolved over 1–4 weeks spontaneously or after topical or systematic steroids. In 1999, Nakao reported another two HTLV-I carriers who were respectively diagnosed with ARN and diabetic retinopathy and showed similar grayish-white PDs along the retinal vessels [18]. As both patients underwent therapeutic vitrectomy, Nakao et al. found intraoperatively that the PDs were loosely adherent to the walls of vessels and could be readily detached and aspirated by a fluted needle without affecting the involved vessels [18]. Notably, analysis of aqueous humor using polymerase chain reaction revealed the presence of DNA of both Varicella-zoster virus (VZV) and HTLV-I. However, the pathomechanism of the perivascular deposits is not clear. Previous histopathologic findings disclosed perivascular cuffing of lymphocytes in the spinal cord and an increased adherence capability of T lymphocytes to vascular endothelial cells in patients with HTLV-I [56, 57]. Therefore, Nakao et al. hypothesized that the T lymphocytes activated by HTLV-I could adhere to and migrate from the endothelium of retinal vessels and proliferate in an autologous or spontaneous way, resulting in the development of perivascular PDs.
ARN is an inflammatory condition causing vitritis and retinitis, with complications commonly including retinal detachment, anterior ischemic optic neuropathy, and central retinal artery occlusion [58]. Mya Thida Ohn et al. reported three ARN cases induced by herpes zoster virus with preretinal deposits, called ‘preretinal inflammatory precipitates’ in their report. The PDs showed three patterns in the three cases, respectively, including a perivascular pattern (resembling deposits in HAU), deposits with thickened posterior hyaloid, and isolated prefoveal deposits (similar to deposits in OT), although there was no laboratory evidence showing HTLV-I or toxoplasmosis infection [20]. Sogawa et al. reported a case with ARN showing perivascular deposits and prefoveal deposits visualized on OCT, which resolved within 6 months after antiviral treatment [19].
Preretinal Deposits in Endogenous Fungal Endophthalmitis
Endogenous fungal endophthalmitis (EFE) is a potentially blinding ocular condition that develops secondary to the hematogenous spread of microbes from distant foci in the body. Most patients with EFE have predisposing risk factors, including diabetes mellitus, chemotherapy, organ transplant, indwelling catheters, intravenous drug abuse, increased estrogen levels in pregnancy, and prolonged corticosteroid and antibiotic use [59]. The majority of cases of endogenous endophthalmitis are caused by fungal infections, and the most common pathogens are Candida species, followed by Aspergillus species [60, 61]. In EFE, the initial sites of ocular infection are the choroid and ciliary body, because of the higher blood perfusion, while the retina and vitreous show a secondary involvement [62]. PDs are also a very common sign in EFE, especially in endogenous Candida endophthalmitis (ECE) [21,22,23,24,25]. In the ophthalmoscope, the PDs manifest as round, creamy yellowish-white lesions. OCT shows the PDs as cloud-like fungal aggregates accompanied by subretinal fluid, subretinal saw-tooth infiltrates, and full-thickness retinal edema. Unlike PDs in other entities, the PDs in ECE have a strong shadowing effect on OCT, and the retinal structures underneath the PDs cannot be observed. The strong shadowing effect indicates more dense biological material in the PDs. In addition, there is clear evidence of PDs from intraretinal or choroidal foci. Invernizzi et al. proposed two patterns of ECE on OCT—an intraretinal pattern (within the inner retinal layers) and a chorioretinal pattern (both retina and choriocapillaris) [23]. In Invernizzi’s study, they documented the spread and infiltration of fungal foci from their intraretinal or choroidal origin toward the vitreous cavity. In other words, when we see PDs in ECE, it means that the fungal lesion has broken through the choroid and retina and has entered a slightly late stage of the disease. In terms of treatment, oral or intravenous antifungal drugs are also necessary to treat the systemic infection. Vitrectomy is superior to blood cultures in confirming the etiology of infection [63]. In most cases, the lesions regress after treatment. However, the penetration of the microorganisms from the choroid through the retinal tissue often results in scar formation.
Preretinal Deposits in Uveitis of Uncertain Etiology
Paulus et al. reported a 6-year-old girl with intermediate uveitis and anterior inflammatory cells presenting with snowballs and PDs in the fovea. Work-up for uveitis showed no abnormalities but a positive TB skin test. However, the vitritis and deposits did not resolve after anti-TB treatment and steroids [14]. Also, Calles Monar et al. reported a case showing round, diffusely scattered PDs with macula edema and peripheral vascular leakage on FFA in a vitrectomized eye. The vitreous fluids showed no evidence of infection. After vitrectomy, the patient was diagnosed with inflammatory spondyloarthritis without sacroiliitis. Part of the deposits and vitritis persisted after treatment [6]. PDs occurring in eyes without certain diagnoses likely have a poor response to conventional anti-inflammatory treatment.
Preretinal Deposits in Silicone Oil Tamponade
SOs are a type of tamponade widely used in pars plana vitrectomy (PPV); they include conventional polydimethylsiloxane (PDMS) silicone oil (CSO), which mainly offers superior tamponade, and heavy silicone oil (HSO), which mostly offers inferior tamponade [64, 65]. During SO tamponade or after SO removal, some researchers have reported that emulsification of SO (both CSO and HSO), which might be due to disturbance of the vitreous microenvironment by inflammation or postoperative remnants and so on, can produce visible oil deposits (namely, droplets) preretinally or even intraretinally [26, 28, 66,67,68,69]. Zewar et al. classified the oil droplets into macrodroplets (or bubbles) and microdroplets (or dots) according to the size and reflectivity of the droplets in the patients after HSO removal. The macrodroplets were defined as droplets ranging between 50 and 300 μm with variably reflective structures, different shapes, and discrete borders, whereas the microdroplets were defined as hyperreflective, minute, spherical materials with separate or continuous borders [29]. Zewar et al. noticed that macrodroplets seem to occur and increase with a longer duration of intraocular SO tamponade because a longer tamponade duration might result in postoperative inflammation development [29, 70]. Some similar observations had previously also been reported for CSO tamponade [28, 68, 69]. The mechanisms of preretinal droplet formation remain elusive. However, some researchers have proposed that intraocular inflammation is the main factor stimulating preretinal droplet development, as evidenced histologically by infiltrates of large cytoplasmic macrophages and silicone vacuoles [28, 66, 71]. On OCT, since the non-emulsified and purified SO is hyporeflective [72], the reflectivity of the oil droplets is heterogeneous due to the different mixed proportions of emulsified SO droplets, fibrotic tissue, and inflammatory infiltrate [28, 29]. The droplets with higher reflectivity probably contain more SO, whereas the isoreflective droplets may suggest a majority of biological materials in the composition [29]. Additionally, Pilli et al. found that the preretinal droplets in three patients with SO tamponade showed hyporeflective darker spots, giving a quasi-pitted appearance resembling the appearance of a strawberry, due to the presence of the retinal surface and the glossy, bright reflection from SO on multicolor and infrared images [30]. In addition to the application of SO tamponade in normal vitrectomy, Sachdeva et al. reported that pigmentary PDs visualized in patients after large relaxing retinectomies and SO tamponade for retinal detachment related to proliferative vitreoretinopathy [27]. These deposits histologically revealed RPE cells with intracellular silicone oil droplets, melanin granules, glial tissue, and fibrous stroma, indicating that RPE cells can phagocytose emulsified oil droplets when these cells are in direct contact with SO in the eyes after retinectomy [27]. All the aforementioned findings indicate that the hyperreflective deposits on OCT in SO tamponade eyes are not composed of emulsified oil alone, but that this oil is present in combination with inflammatory cells, fibrous tissue, melanin, and other biological materials. Complications in the setting of the oil droplets play an important role in visual prognosis. It is reported that the occurrence of cystoid macular edema and epiretinal membrane is statistically associated with preretinal droplets [26, 29]. The droplets were also documented intraretinally, subretinally, beneath the epiretinal membrane, and on the optic nerve [68]. The intraretinal droplets are noteworthy because of their potential vision-threatening effects, and the preretinal droplets might be a source of intraretinal SO, but the underlying mechanisms are uncertain [26, 29, 68, 69]. Patients who have SO tamponade should be considered for the timely possible SO removal once the retina is structurally flat and stable.
Preretinal Deposits in Eyes with Other Exogenous Materials
In addition to SO tamponade, other exogenous materials, including intravitreal antibiotics and unexpected viscoelastic substances egressing into the VC, also form preretinal deposits [31,32,33]. Intravitreal broad-spectrum antibiotic is a recommended treatment for endophthalmitis. Vancomycin and ceftazidime are the common drugs of choice for Gram-positive bacteria and Gram-negative bacteria, respectively [73]. However, there were two cases with PDs after intravitreal vancomycin and ceftazidime for endophthalmitis. Javey et al. reported that a 61-year-old healthy woman presented diffuse white deposits in a preretinal location after intravitreal vancomycin and ceftazidime for endophthalmitis. The PDs appeared as white-appearing PDs consistent with aggregations of concomitantly administered intravitreal vancomycin and ceftazidime on color fundus photograph (CFP). OCT showed hyperreflective deposits with a round top and wide base [31]. Valdes Lara et al. reported a similar case with PDs of a lower height and broader base seen on OCT [32]. The PDs in the two mentioned cases were considered aggregations of vancomycin and ceftazidime. When vancomycin and ceftazidime are mixed in the same syringe, a yellow-white deposit may result, and the same appearance has been described clinically along the needle tract in the VC after treatment [74]. The deposit formation is thought to be due to the alkaline pH of vancomycin (pH 5–7.5) and the presence of bicarbonate in most ceftazidime preparations (pH 2.5–4.5). The deposits in the two mentioned cases completely resolved in 4 weeks without any specific management [31, 32]. In another two cases reported by Behera [33], hydroxypropyl methylcellulose was used as a viscoelastic substance during cataract surgery, and the viscoelastic substance fell into the VC due to the breach of the posterior capsule barrier, resulting in diffusely scattered, round, white deposits on the retina that mimic bacterial colonies. But no evidence of infection could be established, and no intravitreal antibiotics were used. The deposits were strongly presumed to be the viscoelastic substance in combination with some inflammatory infiltration [33].
Differentiating PDs from Kyrieleis Plaques
Of note, PDs have some similarities with Kyrieleis plaques, which were previously referred to as segmental retinal periarteritis. Kyrieleis plaques were first described by Kyrieleis in 1933 in a case with presumed tubercular uveitis (TBU) [75], which is characterized by focal or glistening yellow-white accumulations surrounding retinal arteries, particularly near an area of active retinal infection or inflammation [76]. Kyrieleis plaques share a similar disease spectrum to PDs, including TRC [77, 78], TBU, syphilitic uveitis [79], and VZV and Cytomegalovirus (CMV) infections [80, 81]. Both PDs and Kyrieleis plaques represent inflammatory signs, most of which can be fully recovered from and do not worsen the prognosis. More importantly, they both have a preference for retinal vessels. However, PDs are different from Kyrieleis plaques in the following ways. First, the imaging appearance of PDs is different from that of Kyrieleis plaques. In the ophthalmoscope, PDs are mildly transparent grayish-white or yellowish-white dots, while Kyrieleis plaques are glistening yellowish-white accumulations with high reflectivity of the affected vessel walls. In addition, PDs can involve both retinal arteries and veins, while Kyrieleis plaques affect only the retinal arteries. On fundus autofluorescence (FAF), fundus fluorescence angiography (FFA), and indocyanine green angiography (ICGA), PDs show hypofluorescence throughout all the frames. By contrast, Kyrieleis plaques show increased autofluorescence on FAF and early hypofluorescence and late hyperfluorescence on FFA and ICGA [82]. Second, PDs are located in the interface of the retina and VC, which is confirmed by OCT, whereas the inflammation of Kyrieleis plaques occurs only within the vessel walls, with no involvement seen outside the vessel walls [82]. Third, PDs can completely resolve without structural impairment after treatment, in contrast to Kyrieleis plaques, which potentially persist clinically with perivascular sheathing in some patients, despite the resolution of the chorioretinitis after antibiotic treatment [82].
In summary, to better elucidate the diagnostic approach to PD, we constructed a mind map based on our review (Fig. 2).
Conclusions
Our literature review reveals that PDs in different entities may have something in common. First, regardless of the PDs from exogenous materials, PDs occur more often in inflammatory conditions related to infection (i.e., OT, syphilitic uveitis, ARN, and EFE) rather than in non-infectious conditions. In addition, in infectious inflammatory conditions, the PDs are commonly accompanied by a retinitis area, especially in syphilis uveitis, where the PDs are above the retinitis area. Based on the findings of the literature review, we assume that PDs in inflammatory conditions are likely to have two sources—the retinitis area and the VC. The retinitis area could produce retinal tissue debris and inflammatory cells, resulting in the development of PDs that adhere to the retinitis area in some cases. Second, the presence of PDs is a predictor suggestive of the active state of the disease, probably providing suggestive information for treatment. Third, PDs have a good prognosis in either inflammatory or exogenous conditions. The PDs gradually resolve in 1–4 weeks once the etiological treatment is given, indicating that the PDs are not serious blinding manifestations. Regarding exogenous materials, surgical removal is the first option, whereas antibiotic aggregates could resolve spontaneously. Finally, concerning the nature of the PDs, it is acknowledged in most of the literature that the PDs consist of inflammatory cells or white blood cells except for conditions related to exogenous materials. The mechanisms of deposit development are unclear, but inflammation seems to be the main contributing factor in most entities. From cytopathological evidence in the diagnosis of VRL, we conclude that the PDs in VRL are likely to mainly comprise lymphoma cells, especially the large B-cell type according to our review. However, because of the absence of cytopathological evidence in other inflammatory diseases, the precise composition is elusive and may vary between entities. Despite the rarity of this condition, further investigations should be conducted to reveal the underlying mechanisms.
References
Nakao K, Ohba N. HTLV-I associated uveitis revisited: characteristic grey-white, granular deposits on retinal vessels. Br J Ophthalmol. 1996;80:719–22. https://doi.org/10.1136/bjo.80.8.719.
Guagnini AP, De Potter P, Levecq L, Kozyreff A. Atypical spherical deposition on vitreoretinal interface associated with toxoplasmic chorioretinitis. Graefes Arch Clin Exp Ophthalmol. 2007;245:158–60. https://doi.org/10.1007/s00417-006-0330-6.
Goldenberg D, Goldstein M, Loewenstein A, Habot-Wilner Z. Vitreal, retinal, and choroidal findings in active and scarred toxoplasmosis lesions: a prospective study by spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013;251:2037–45. https://doi.org/10.1007/s00417-013-2334-3.
Invernizzi A, Agarwal AK, Ravera V, Mapelli C, Riva A, Staurenghi G, McCluskey PJ, Viola F. Comparing optical coherence tomography findings in different aetiologies of infectious necrotising retinitis. Br J Ophthalmol. 2018;102:433–7. https://doi.org/10.1136/bjophthalmol-2017-310210.
Yonekawa Y, Abbey AM, van Laere L, Shah AR, Thomas BJ, Ruby AJ, Faia LJ. Stalagmite-like preretinal inflammatory deposits in vitrectomized eyes with posterior uveitis. Digit J Ophthalmol. 2017;23:18–22. https://doi.org/10.5693/djo.02.2016.02.002.
Calles Monar PS, Sanabria Ruiz-Colmenares MR, Cano Suárez MT, García de Arriba S, Alonso Tarancón AM, Villoria Díaz S. Stalagmite-like pre-retinal deposits in the optical coherence tomography of two vitrectomy patients with panuveitis. Arch Soc Esp Oftalmol (Engl Ed). 2022;97:104–8. https://doi.org/10.1016/j.oftale.2020.12.015.
Oliver GF, Ferreira LB, Vieira BR, Arruda S, Araújo M, Carr JM, Smith JR, Furtado JM. Posterior segment findings by spectral-domain optical coherence tomography and clinical associations in active toxoplasmic retinochoroiditis. Sci Rep. 2022;12:1156. https://doi.org/10.1038/s41598-022-05070-9.
Ksiaa I, Khochtali S, Mefteh M, Ben Fredj M, Ben Amor H, Abroug N, Khairallah M. Distinguishing swept-source optical coherence tomography findings in active toxoplasmic retinochoroiditis. Eye (Lond). 2022;36:1222–30. https://doi.org/10.1038/s41433-021-01491-4.
Reddy S, Cunningham ET Jr, Spaide RF. Syphilitic retinitis with focal inflammatory accumulations. Ophthalmic Surg Lasers Imaging. 2006;37:429–31. https://doi.org/10.3928/15428877-20060901-13.
Fu EX, Geraets RL, Dodds EM, Echandi LV, Colombero D, McDonald HR, Jumper JM, Cunningham ET Jr. Superficial retinal precipitates in patients with syphilitic retinitis. Retina. 2010;30:1135–43. https://doi.org/10.1097/IAE.0b013e3181cdf3ae.
Yang P, Zhang N, Li F, Chen Y, Kijlstra A. Ocular manifestations of syphilitic uveitis in Chinese patients. Retina. 2012;32:1906–14. https://doi.org/10.1097/IAE.0b013e3182509796.
Rodrigues RA, Nascimento HM, Muccioli C. Yellowish dots in the retina: a finding of ocular syphilis? Arq Bras Oftalmol. 2014;77:324–6. https://doi.org/10.5935/0004-2749.20140081.
Horng CT, Tsai PF, Tsai ML. Multiple preretinal yellowish dots in a patient with syphilis. Ci Ji Yi Xue Za Zhi. 2018;30:255–6. https://doi.org/10.4103/tcmj.tcmj_79_18.
Paulus YM, Wong IG, Sanislo S, Moshfeghi DM. Prefoveal vitreous condensation in chronic inflammation. Ophthalmic Surg Lasers Imaging Retina. 2014;45:447–50. https://doi.org/10.3928/23258160-20140806-03.
Saito T, Ohguro N, Iwahashi C, Hashida N. Optical coherence tomography manifestations of primary vitreoretinal lymphoma. Graefes Arch Clin Exp Ophthalmol. 2016;254:2319–26. https://doi.org/10.1007/s00417-016-3395-x.
Yang X, Dalvin LA, Mazloumi M, Ferenczy S, Lim LS, Ancona-Lezama D, Shields JA, Mashayekhi A, Shields CL. Spectral domain optical coherence tomography features of vitreoretinal lymphoma in 55 eyes. Retina. 2021;41:249–58. https://doi.org/10.1097/iae.0000000000002819.
Zhou X, Tian S, Zhou X, Shi H, Li Y, Xiao J, Chen K, Chen B, Xu G, Wang Q. Optical coherence tomography benefits the diagnosis and follow-up of primary central nervous system lymphoma with intraocular involvement. Cancer Manag Res. 2022;14:1007–18. https://doi.org/10.2147/cmar.S353142.
Nakao K, Ohba N, Uemura A, Okubo A, Sameshima M, Hayami K. Gray-white, spherical deposition on retinal vessel associated with acute retinal necrosis and diabetic retinopathy in HTLV-I carriers. Jpn J Ophthalmol. 1998;42:490–4. https://doi.org/10.1016/s0021-5155(98)00043-4.
Sogawa T, Hashida N, Sawa M, Nishida K. Rare association of perivascular granulomatous lesions in a patient with acute retinal necrosis. Case Rep Ophthalmol. 2015;6:373–9. https://doi.org/10.1159/000442084.
Ohn MT, Vishnubala A, Hughes PW, Tun HN, Raja M, Goldsmith C, Burton B. Pre-retinal inflammatory precipitates (PIP) in three cases of acute retinal necrosis caused by herpes zoster virus. EC Ophthalmol. 2019;10:131–5.
Imago M, Imai H, Nakanishi Y, Azumi A. Optical coherence tomography for monitoring the process of Candida endophthalmitis. Acta Ophthalmol. 2009;87:680–2. https://doi.org/10.1111/j.1755-3768.2008.01297.x.
Cho M, Khanifar AA, Chan RV. Spectral-domain optical coherence tomography of endogenous fungal endophthalmitis. Retin Cases Brief Rep. 2011;5:136–40. https://doi.org/10.1097/ICB.0b013e3181cc2146.
Invernizzi A, Symes R, Miserocchi E, Cozzi M, Cereda M, Fogliato G, Staurenghi G, Cimino L, McCluskey P. Spectral domain optical coherence tomography findings in endogenous candida endophthalmitis and their clinical relevance. Retina. 2018;38:1011–8. https://doi.org/10.1097/iae.0000000000001630.
Zhuang H, Ding X, Gao F, Zhang T, Ni Y, Chang Q, Xu G. Optical coherence tomography features of retinal lesions in Chinese patients with endogenous Candida endophthalmitis. BMC Ophthalmol. 2020;20:52. https://doi.org/10.1186/s12886-020-01337-9.
Anvari P, Mirshahi R, Sedaghat A, Ghasemi Falavarjani K. “Inverted snowing-cloud” sign in endogenous Candida endophthalmitis. J Ophthalmic Vis Res. 2022;17:303–5. https://doi.org/10.18502/jovr.v17i2.10807.
Odrobina D, Laudańska-Olszewska I. Analysis of the time and location of the silicone oil emulsification by spectral-domain optical coherence tomography after silicone oil tamponade. Biomed Res Int. 2014;2014: 372045. https://doi.org/10.1155/2014/372045.
Sachdeva MM, Jakobiec FA, Stagner AM, Papakostas A, Eliott D. Clinical and ultrastructural studies of epiretinal pigmentary deposits after retinectomy with silicone oil. Ophthalmology. 2016;123:2595–602. https://doi.org/10.1016/j.ophtha.2016.08.049.
Trivizki O, Zur D, Goldenberg D, Rabina G, Fleissig E, Barak A, Shulman S, Loewenstein A, Schwartz S. A novel finding of hyperreflective material in the silicone-retina interface: an optical coherence tomographic and histopathological study. Retina. 2020;40:2055–60. https://doi.org/10.1097/iae.0000000000002691.
Zewar AN, Lochhead J. Optical coherence tomographic patterns of posterior segment sticky heavy silicone oil. Retina. 2021;41:2556–63. https://doi.org/10.1097/iae.0000000000003254.
Pilli S, Murjaneh S. Strawberry retina: silicone oil—retina interface on multimodal imaging. Retin Cases Brief Rep. 2021. https://doi.org/10.1097/icb.0000000000001188.
Javey G, Albini TA, Moshfeghi AA, Kelly KT, Flynn HW Jr. Spectral-domain optical coherence tomography appearance of preretinal aggregation of intravitreal antibiotics. Retina. 2010;30:184–5. https://doi.org/10.1097/IAE.0b013e3181bfbdc9.
Valdes Lara CA, Testi I, Pavesio C. Ceftazidime and vancomycin deposits after intravitreal injection in a vitrectomized eye. Ophthalmology. 2021;128:1591. https://doi.org/10.1016/j.ophtha.2021.04.010.
Behera UC, Sivakumar RR, Prajna L, Agrawal N, Rajan RP. Epiretinal deposits post cataract extraction. Retin Cases Brief Rep. 2013;7:359–61. https://doi.org/10.1097/ICB.0b013e3182964f91.
Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, Androudi S. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2018;26:2–16. https://doi.org/10.1080/09273948.2016.1196713.
Kijlstra A, Petersen E. Epidemiology, pathophysiology, and the future of ocular toxoplasmosis. Ocul Immunol Inflamm. 2014;22:138–47. https://doi.org/10.3109/09273948.2013.823214.
Gass JMD. Stereoscopic atlas of macular diseases: diagnosis and treatment. St Louis: Mosby; 1997.
Nijhawan R, Bansal R, Gupta N, Beke N, Kulkarni P, Gupta A. Intraocular cysts of Toxoplasma gondii in patients with necrotizing retinitis following periocular/intraocular triamcinolone injection. Ocul Immunol Inflamm. 2013;21:396–9. https://doi.org/10.3109/09273948.2013.810276.
Furtado JM, Simões M, Vasconcelos-Santos D, Oliver GF, Tyagi M, Nascimento H, Gordon DL, Smith JR. Ocular syphilis. Surv Ophthalmol. 2022;67:440–62. https://doi.org/10.1016/j.survophthal.2021.06.003.
Dutta Majumder P, Chen EJ, Shah J, Ching Wen Ho D, Biswas J, See Yin L, Gupta V, Pavesio C, Agrawal R. Ocular syphilis: an update. Ocul Immunol Inflamm. 2019;27:117–25. https://doi.org/10.1080/09273948.2017.1371765.
Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25:513–8. https://doi.org/10.1097/icu.0000000000000099.
Tsan GL, Claiborne RT. Ocular syphilis. Clin Exp Optom. 2021;104:756–9. https://doi.org/10.1080/08164622.2021.1906848.
Meyer CH, Silva-Papi M, Mennel S. Chorioretinitis of uncertain origin in a young man. Ophthalmologe. 2004;101:518–20. https://doi.org/10.1007/s00347-003-0871-6.
Pichi F, Neri P. Multimodal imaging patterns of posterior syphilitic uveitis: a review of the literature, laboratory evaluation and treatment. Int Ophthalmol. 2020;40:1319–29. https://doi.org/10.1007/s10792-020-01285-9.
Levasseur SD, Wittenberg LA, White VA. Vitreoretinal lymphoma: a 20-year review of incidence, clinical and cytologic features, treatment, and outcomes. JAMA Ophthalmol. 2013;131:50–5. https://doi.org/10.1001/jamaophthalmol.2013.569.
Chan CC, Sen HN. Current concepts in diagnosing and managing primary vitreoretinal (intraocular) lymphoma. Discov Med. 2013;15:93–100.
Rothova A, Ooijman F, Kerkhoff F, Van Der Lelij A, Lokhorst HM. Uveitis masquerade syndromes. Ophthalmology. 2001;108:386–99. https://doi.org/10.1016/s0161-6420(00)00499-1.
Chan CC, Fischette M, Shen D, Mahesh SP, Nussenblatt RB, Hochman J. Murine model of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2005;46:415–9. https://doi.org/10.1167/iovs.04-0869.
Chan CC, Shen D, Hackett JJ, Buggage RR, Tuaillon N. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110:421–6. https://doi.org/10.1016/s0161-6420(02)01737-2.
Keino H, Okada AA, Watanabe T, Echizen N, Inoue M, Takayama N, Nagane M. Spectral-domain optical coherence tomography patterns in intraocular lymphoma. Ocul Immunol Inflamm. 2016;24:268–73. https://doi.org/10.3109/09273948.2014.1002568.
Egawa M, Mitamura Y, Hayashi Y, Naito T. Spectral-domain optical coherence tomographic and fundus autofluorescence findings in eyes with primary intraocular lymphoma. Clin Ophthalmol. 2014;8:335–41. https://doi.org/10.2147/opth.S58114.
Coffin JM. The discovery of HTLV-1, the first pathogenic human retrovirus. Proc Natl Acad Sci USA. 2015;112:15525–9. https://doi.org/10.1073/pnas.1521629112.
Ikeda E, Ono A, Hikita N, Arima K, Mochizuki M, Yamaguchi K, Tajima K, Kiyokawa H. Estimated prevalence rate of HTLV-I uveitis in Chikugo. Nippon Ganka Gakkai Zasshi. 1998;102:327–32.
Nakao K, Ohba N, Matsumoto M. Noninfectious anterior uveitis in patients infected with human T-lymphotropic virus type I. Jpn J Ophthalmol. 1989;33:472–81.
Kamoi K, Watanabe T, Uchimaru K, Okayama A, Kato S, Kawamata T, Kurozumi-Karube H, Horiguchi N, Zong Y, Yamano Y, Hamaguchi I, Nannya Y, Tojo A, Ohno-Matsui K. Updates on HTLV-1 uveitis. Viruses. 2022. https://doi.org/10.3390/v14040794.
Ohba N, Nakao K, Isashiki Y, Kaminagayoshi T, Sonoda S, Yashiki S, Osame M. Clinical features of HTLV-I associated uveitis determined in multicenter collaborative study. Study Group for HTLV-I Associated Ocular Diseases. Jpn J Ophthalmol. 1994;38:168–74.
Akizuki S, Nakazato O, Higuchi Y, Tanabe K, Setoguchi M, Yoshida S, Miyazaki Y, Yamamoto S, Sudou S, Sannomiya K, et al. Necropsy findings in HTLV-I associated myelopathy. Lancet. 1987;1:156–7. https://doi.org/10.1016/s0140-6736(87)91984-2.
Tsukada N, Miyagi K, Matsuda M, Yanagisawa N. Increased levels of circulating intercellular adhesion molecule-1 in multiple sclerosis and human T-lymphotropic virus type I-associated myelopathy. Ann Neurol. 1993;33:646–9. https://doi.org/10.1002/ana.410330614.
Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114:756–62. https://doi.org/10.1016/j.ophtha.2006.08.037.
Chen KJ, Wu WC, Sun MH, Lai CC, Chao AN. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol. 2012;154:213–4. https://doi.org/10.1016/j.ajo.2012.03.016. (author reply 214).
Zhang H, Liu Z. Endogenous endophthalmitis: a 10-year review of culture-positive cases in northern China. Ocul Immunol Inflamm. 2010;18:133–8. https://doi.org/10.3109/09273940903494717.
Sheu SJ. Endophthalmitis. Korean J Ophthalmol. 2017;31:283–9. https://doi.org/10.3341/kjo.2017.0036.
Bajor A, Luhr A, Brockmann D, Suerbaum S, Framme C, Sedlacek L. Listeria monocytogenes endophthalmitis—case report and review of risk factors and treatment outcomes. BMC Infect Dis. 2016;16:332. https://doi.org/10.1186/s12879-016-1680-2.
Spelta S, Di Zazzo A, Antonini M, Bonini S, Coassin M. Does endogenous endophthalmitis need a more aggressive treatment? Ocul Immunol Inflamm. 2021;29:937–43. https://doi.org/10.1080/09273948.2019.1705497.
Wolf S, Schön V, Meier P, Wiedemann P. Silicone oil-RMN3 mixture (“heavy silicone oil”) as internal tamponade for complicated retinal detachment. Retina. 2003;23:335–42. https://doi.org/10.1097/00006982-200306000-00008.
MacGregor C, Jonas A, Hanifudin A, Lochhead J. Ratios for double silicone oil endotamponade—in vitro observations may assist with ratio selection. BMC Ophthalmol. 2017;17:264. https://doi.org/10.1186/s12886-017-0660-7.
Russo A, Morescalchi F, Donati S, Gambicorti E, Azzolini C, Costagliola C, Semeraro F. Heavy and standard silicone oil: intraocular inflammation. Int Ophthalmol. 2018;38:855–67. https://doi.org/10.1007/s10792-017-0489-3.
Haridas AS, Jayaprakasam A, Somanathan S, Soni M. Spectral domain OCT features of intraretinal silicone oil in eyes following oil removal. Investig Ophthalmol Vis Sci. 2010;51:1755.
Errera MH, Liyanage SE, Elgohary M, Day AC, Wickham L, Patel PJ, Sahel JA, Paques M, Ezra E, Sullivan PM. Using spectral-domain optical coherence tomography imaging to identify the presence of retinal silicone oil emulsification after silicone oil tamponade. Retina. 2013;33:1567–73. https://doi.org/10.1097/IAE.0b013e318287d9ea.
Cebeci Z, Sadik MT, Ogurel MB, Altinkurt E, Kir N. Evaluation of emulsified silicone oil with spectral domain-optical coherence tomography and fluorescein angiography. Int Ophthalmol. 2020;40:2267–74. https://doi.org/10.1007/s10792-020-01409-1.
Dresp JH, Menz DH. The phenomenon of “sticky” silicone oil. Graefes Arch Clin Exp Ophthalmol. 2007;245:863–8. https://doi.org/10.1007/s00417-006-0450-z.
Carpineto P, Angelucci D, Nubile M, Colasante A, Di Antonio L, Ciancaglini M, Mastropasqua L. Immunohistochemical findings in epiretinal membrane after long-term silicone oil tamponade: case report. Eur J Ophthalmol. 2006;16:887–90. https://doi.org/10.1177/112067210601600621.
Ghoraba HH, Zaky AG, Abd Al Fatah HM, El Gemai E, Heikal MA. Sticky silicone oil. Retina. 2017;37:1599–606. https://doi.org/10.1097/iae.0000000000001377.
Roth DB, Flynn HW Jr. Antibiotic selection in the treatment of endophthalmitis: the significance of drug combinations and synergy. Surv Ophthalmol. 1997;41:395–401. https://doi.org/10.1016/s0039-6257(97)00005-2.
Lifshitz T, Lapid-Gortzak R, Finkelman Y, Klemperer I. Vancomycin and ceftazidime incompatibility upon intravitreal injection. Br J Ophthalmol. 2000;84:117–8. https://doi.org/10.1136/bjo.84.1.117a.
Kyrieleis W. Discontinuous reversible arteriopathy in uveitis. Albrecht Von Graefes Arch Ophthalmol. 1950;150:600–13.
Griffin AO, Bodian M. Segmental retinal periarteritis; a report of three cases. Am J Ophthalmol. 1959;47:544–8.
Chazalon E, Conrath J, Ridings B, Matonti F. Kyrieleis arteritis: report of two cases and literature review. J Fr Ophtalmol. 2013;36:191–6. https://doi.org/10.1016/j.jfo.2012.03.014.
Mahjoub A, Ben Abdesslem N, Zaafrane N, Sellem I, Sahraoui F, Nouri H, Hadj RB, Ben Alaya A, Krifa F, Hachemi M. Kyrieleis arteritis associated with toxoplasmic retinochoroiditis: a case report. Ann Med Surg (Lond). 2022;78: 103802. https://doi.org/10.1016/j.amsu.2022.103802.
Krishnamurthy R, Cunningham ET Jr. Atypical presentation of syphilitic uveitis associated with Kyrieleis plaques. Br J Ophthalmol. 2008;92:1152–3. https://doi.org/10.1136/bjo.2007.124693.
Empeslidis T, Konidaris V, Brent A, Vardarinos A, Deane J. Kyrieleis plaques in herpes zoster virus-associated acute retinal necrosis: a case report. Eye (Lond). 2013;27:1110–2. https://doi.org/10.1038/eye.2013.110.
Patel A, Pomykala M, Mukkamala K, Gentile RC. Kyrieleis plaques in cytomegalovirus retinitis. J Ophthalmic Inflamm Infect. 2011;1:189–91. https://doi.org/10.1007/s12348-011-0033-y.
Pichi F, Veronese C, Lembo A, Invernizzi A, Mantovani A, Herbort CP, Cunningham ET Jr, Morara M, Ricci F, Neri P, Nucci P, Ciardella AP, Staurenghi G, Lowder CY, Srivastava SK. New appraisals of Kyrieleis plaques: a multimodal imaging study. Br J Ophthalmol. 2017;101:316–21. https://doi.org/10.1136/bjophthalmol-2015-308246.
Acknowledgements
We acknowledge the contribution and support of the Department of Ophthalmology, Beijing Tongren Hospital, Capital Medical University for providing imaging of the patient.
Funding
The journal’s Rapid Service fee and study were supported by the National Natural Science Foundation of China (82171073). The funding organizations had no role in the design or conduct of this research.
Author Contributions
Writing and draft preparation: Yizhe Cheng. Review of the literature: Yizhe Cheng and Chunli Chen. Revisions: Chunli Chen, Xiaoyan Peng, and Zhihan Zhang. Conceptualization: Yizhe Cheng. Review and editing: Xiaoyan Peng. All authors have read and approved the manuscript.
Disclosures
The authors have no proprietary or commercial interest in any drugs and equipment used in this article. All named authors confirm that they have no competing interests to declare.
Compliance with Ethics Guidelines
The Ethics Committee of the Beijing Tongren Hospital affiliated to Capital Medical University waived the need for formal approval in this study given the nature of the study. Full consent for the procedures described in Fig. 1 was obtained from the patient.
Data Availability
All data supporting our findings are contained within the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cheng, Y., Chen, C., Zhang, Z. et al. Clinical Features and Possible Origin of Preretinal Deposits in Different Ocular Diseases and Events: A Narrative Review. Ophthalmol Ther 12, 737–753 (2023). https://doi.org/10.1007/s40123-023-00674-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00674-4