Abstract
Introduction
The objective of this study was to compare the microbiome in the aqueous humour and gut of people with diabetes mellitus (DM) with and without diabetic retinopathy (DR).
Methods
This was a prospective controlled study. The study included 17 people undergoing intraocular surgery in their naïve eyes. Stool samples were obtained in the perioperative period; aqueous humour samples of sufficient quantity were obtained in 12 people during intraocular surgery. Dietary information was obtained using a previously validated questionnaire. The gut and aqueous humour samples were assessed for microbiome using 16S rRNA gene sequencing coupled with QIIME and R software.
Results
Aqueous humour was analysed in 12 people: 4 each healthy controls, people with DM, and people with DR. There were minor differences at the phyla levels, but the aqueous humour microbiomes of healthy controls, DM, and DR formed three distinct clusters on heat map analysis with discriminatory genera. This genera-level clustering was more apparent for the intraocular than the gut microbiome. In people with DM and DR, we identified genera unique to the eye or the gut. There was a consistent reduction in the abundance of anti-inflammatory bacteria in people with DR than DM.
Conclusions
There is a difference in intraocular and gut microbiome regardless of disease or health. Our preliminary findings indicate distinctive features of the intraocular microbiome in people with DR compared with those without it. While this distinctiveness appears more evident in aqueous humour than in the gut, it needs further confirmation with larger studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This is a pilot assessment of aqueous humour and gut samples obtained from three groups: diabetes mellitus (DM), diabetic retinopathy (DR), and control. |
The heat maps developed showed distinctive clustering of the groups with discriminatory genera in aqueous humour, indicating differential microbiomes. |
Genera unique to the gut or the aqueous humour could be identified in DM and DR groups, raising causative implications. |
DR group showed a consistent reduction in anti-inflammatory bacteria. |
Inter-group differences were more pronounced in aqueous humour of DR group than DM group. |
Introduction
Diabetes mellitus (DM) is a leading cause of disability due to its long-term complications. The proportion of disability-adjusted life years (DALY) due to DM has increased by 146% in the past three decades globally [14]. The care for these complications would require an estimated 2.2% of the world’s gross domestic product by 2030 [5]. Diabetic retinopathy (DR) is the most common microvascular complication of DM, with an age-adjusted prevalence of 34% worldwide [36]. Due to the long latency between the occurrence of DR and the development of sight-threatening DR (STDR), prevention and early management of DR is considered an effective method of reducing the DR-related burden of visual impairment [7, 8, 35].
Multiple pathophysiological pathways have been considered for DR until the last decade. These have been directed at ischemia-related damage to the retinal vascular barrier, retinal inflammation and retinal neurodegeneration [30]. Recent research has indicated a role of the gut microbiome, which is currently referred to as the “gut-retinal axis” in DR [7, 8]. This hypothesis begins with implicating gut dysbiosis for its causal role in people with DM. The mechanisms include a permeable gut barrier, altered inflammatory cascades, altered glucose metabolism and insulin resistance that impacts retinal neurons [16]. Incidentally, the same mechanisms also affect DR. Subsequently, several pre-clinical studies have evaluated and demonstrated an interaction between oral hypoglycaemic agents, gut microbiome and the severity of DM [16, 28] However, there is minimal literature on the direct relationship between the gut and the retina in DR.
Progress in the understanding of the gut–retina axis in DR and the impact of the microbiota on DM is largely due to advanced and high-throughput genetic sequencing techniques that allow for the quick identification among many microorganisms [7, 8]. Beli et al. have shown beneficial preclinical evidence of intermittent fasting-led changes on the gut microbiome in DR [4]. They have also evaluated the possibility of attenuating DR by targeting neuronal–retinal relays induced by gut dysbiosis [4, 33]. Our pre-clinical murine model has identified changes in the microbiota of diabetic and healthy rats. We detected overlaps between diabetic rats with and without DR [26, 27]. Further, we have also noted dysbiosis (measured as abundance and diversities) of both bacteria (gut microbiome) and fungi (gut mycobiome) in people with DM with and without DR, compared with the healthy controls [7, 8, 19]. Similarly, Huang et al. have reported the possibility of using the gut microbiome as a biomarker to distinguish people with DR from those without it [17]. One study from India has shown lesser differentiation in an abundance of microbes, though the authors suggested that the altered proportion of microbes could be predictive of advanced DR [22]. Differences in the genomes, therapy of DM and dietary habits influence gut dysbiosis [7, 8, 16, 17, 19]. These studies have arguably generated considerable interest and some evidence into a possible independent gut–retina axis that influences DR. While multiple mechanisms have been proposed to explain this axis (discussed later) [17], a direct link is missing.
The gut–retinal axis could be a possible target of preventive therapy for DR [13], having shown evidence at the beginning of the axis, the terminal impact on the eye is largely unknown. We are unsure if the permeable gut of people with DM allows microbial invasion of the eye or the retina and thus modulates DR. The current study attempts to answer this question. We analysed the microbiomes of the intraocular fluid (aqueous humour) and gut of people with DM with or without DR and compared these with the healthy controls. We also studied the impact of diet-related factors on the gut–retina axis in people with DM.
Methods
Study Design and Recruitment of Patients
This was a prospective controlled study conducted at a tertiary eye care centre in India. The study protocol was reviewed by the institutional review board, and ethical clearance was obtained (LEC-BHR-P-04-20-426). A total of 17 people were enrolled in the study, informed consent was obtained before enrolment, and it adhered to the tenets of the Declaration of Helsinki.
The participants and controls were recruited from the outpatient department of the Vitreo-retina services. All these patients were planned for intraocular surgery, either for cataract or retinal disorders. Patients with prior history of systemic diseases other than DM and hypertension were excluded. Patients with a history of open globe injury, past ocular surgery, ocular infections and inflammation in the involved eye were also excluded. None of these patients had received topical or systemic antibiotics or intravitreal injections in the past 3 months. The patients were recruited in three pre-planned groups. Group 1 included patients with DM and DR (DR, n = 7); Group 2 included patients with DM but not DR (DM, n = 4), and Group 3 consisted of systemically healthy controls (n = 6). All patients underwent a comprehensive eye examination with electronic documentation of clinical findings before and after surgery. The clinical diagnosis of DR was made with ophthalmoscopy and fundus photography performed by a single retinal surgeon. Blood samples were drawn, and stool samples were collected from all 17 participants within 7 days of surgery. Metabolic profiles inclusive of fasting and random blood glucose (mg/dl), HbA1c (%), blood pressure (mmHg), lipid profile, and renal function tests were assessed for all the patients. None of the patients received antibiotics prior to the surgery.
Diet Scores
A dietary questionnaire in vernacular language was used to collect the dietary habits of the subjects using a weekly recall method based on version 1 of the UK Diabetes and Diet questionnaire, which has been validated in South Asia [11, 12]. Briefly, the diet habits were assigned scores by quantity in diet, frequency, and usage pattern from the perspective of DM. Participants were assigned to healthy, less healthy, or unhealthy habits on a scoring scale of 1–20.
Ocular and Gut Sampling
A single surgeon drew all ocular fluid samples. All surgeries were performed under standard sterile precautions. All sampling was performed as the first step of the surgery. Anterior chamber samples were drawn using a 26G needle capped on a Tuberculin syringe introduced into the anterior chamber and carefully kept over the iris plane. Around 0.05–0.1 ml of aqueous humour was recovered from all subjects; however, an adequate volume could be obtained only from 12 participants (human control, n = 4; DM, n = 4; DR, n = 4). The samples were immediately transferred to an icebox and transported to the laboratory (in the same building) under sterile precautions. Stool samples were collected from all participants and immediately stored at −80 °C until further use.
DNA Extraction from Intraocular and Gut Samples
Using the QIAamp DNA Mini kit and following the protocol for “DNA Purification from Blood or Body Fluids (Spin Protocol)”, genomic DNA was extracted from the intraocular fluids. Genomic DNA was isolated from approximately 150 mg of homogenized faecal sample, and the quality of DNA was checked following the protocols described in our earlier studies [7, 8, 18, 20, 21]. Negative controls (reagents of the respective isolation protocols without the samples) were also processed simultaneously.
Preparation of Illumina Library and Bacterial Microbiome Sequencing
Libraries were prepared by amplifying the V3–V4 region of the 16S rRNA gene using fusion primer, followed by index PCR with Nextera XT index kit (Illumina). The quality of the final libraries was checked with 2200 TapeStation (Agilent) using a high-sensitivity DIOOO screen tape, and later quantified in Qubit Fluorometer. Finally, the sequencing of the libraries was performed in Novaseq 6000 (Illumina) with a paired-end, 2 × 250 bp chemistry at the National Institute of Biomedical Genomics (NIBMG, CoTERI), Kalyani, West Bengal, India.
Taxonomic Classification of Sequenced Reads
Demultiplexing and merging of the paired-end reads was performed using FLASH (https://ccb.jhu.edu/software/FLASH/), and the low-quality reads (mean Phred score < 25) were identified and filtered using PRINSEQ-lite (http://prinseq.sourceforge.net/). Later chimeric sequences were removed using the Usearch61 tool (http://www.drive5.com/usearch/download.html), and only high-quality reads (HQ) were used for picking the operational taxonomic units (OTU) in Quantitative Insights Into Microbial Ecology (QIIME).
Bacteria were identified at 97% similarity with SILVA (Release 138 SSU) reference OTU database, and the taxonomy was assigned as described in our earlier studies [7, 8, 29]. Contamination was removed from all sequences using the functions remove.count, remove.thresh and decon.diff in microDecon [25]. Sparse OTUs (< 0.001% of the total HQ reads) were not considered for further analysis.
Diversity Analyses of Bacterial Microbiomes
Vegan 2.6–2 package in R (http://vegan.r-forge.r-project.org/) was used to generate rarefaction curves and box plots representing alpha diversity indices (Shannon diversity, Simpson index, observed number of OTUs and Chao1).
Statistical Analysis
The microbiome data were analysed using R statistical software, version 4.2.0 (http://cran.r-project.org/, available in the public domain) (R Core Team, 2020).
Identification of Differentially Abundant Genera
Significantly different genera (P < 0.05) among the healthy controls, DM and DR cohorts of the aqueous humour and gut microbiomes were identified by Wilcoxon signed-rank and Kruskal–Wallis tests in R. Distribution of the differentially abundant bacterial genera in the respective cohorts was represented as box plots and two-dimensional heat map (with rank-normalized abundances) in R.
Bacterial Genera Interaction Networks
Networks indicating the interactions among the bacterial genera were generated separately for healthy controls, DM and DR microbiomes using CoNet (https://raeslab.org/software/conet.html) app in Cytoscape (https://cytoscape.org/) program.
Results
A total of 12 patients were included in the descriptive analysis where paired ocular and gut samples could be obtained. The mean age of the participants was 61.16 ± 11.95 years (range 43–90 years). The mean age of people in the control, DM and DR groups was 62.25, 68.75, and 52.5 years, respectively. The mean duration of DM was 9.5 ± 4.8 years (range 0–17 years); it was 14.3 years in the DR group and 4.8 years in the DM group. A total of 8 of the 12 participants were non-vegetarian by diet, 3 each in the healthy control and DR group and 2 in the DM group. Most of the participants (n = 7) had healthy diet scores ≥ 15; three in the healthy control group and one each in DR and DM groups had < 15 healthy scores (Supplementary Table 1).
Analysis of OTUs of the Bacterial Microbiomes from Aqueous Humour of Control, People with Diabetes Mellitus and Diabetic Retinopathy
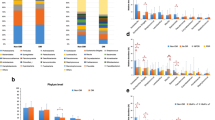
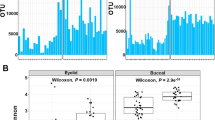
Average HQ reads per microbiome from aqueous humour of the control, DM and DR cohorts were 734,382, 891,164, and 795,636, respectively. From these microbiomes, we identified 937 OTUs (639 references and 298 de novo OTUs). Adequacy of sequencing depth and coverage to capture the total bacterial diversity in the 12 microbiomes is indicated by the saturation of the rarefaction curves (Fig. 1A). Alpha diversity analysis indicated that the observed number of OTUs and Chao1 indices were significantly different (p < 0.05) between DM and DR cohorts than the control. However, there was no significant difference among the groups in Shannon diversity and Simpson indices (Fig. 1B).
A Rarefaction curves of microbiomes obtained from aqueous humour in individuals in the healthy (HC, n = 4), diabetic (DM, n = 4) and diabetic retinopathy (DR, n = 4) groups. B Box plots depicting Alpha diversity indices. *Indicates that the observed number of OTUs and Chao1 indices are significant between DM and DR microbiomes
Bacterial Community Composition
In the bacterial microbiomes from the aqueous humour, 15 different phyla, excluding unclassified phyla, were detected (Table 1). Phyla Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Spirochaetes, Cyanobacteria, Verrucomicrobia, Tenericutes, Elusimicrobia and Chloroflexi had > 0.50% abundance; most abundant among them were Bacteroidetes and Firmicutes (Fig. 2 and Table 1). Differences in the abundance of phyla were not significant among the cohorts except for Tenericutes, which was significantly different in the DM and DR groups (Table 1).
A Phyla in the bacterial microbiomes from aqueous humour of individuals in control (HC, n = 4), diabetic (DM, n = 4) and diabetic retinopathy (DR, n = 4) groups. B Mean abundance of bacterial phyla from aqueous humour microbiomes of individuals in control (HC, n = 4), diabetic (DM, n = 4) and diabetic retinopathy (DR, n = 4) groups
At the genus level, 122, 145, and 93 genera were identified in individuals in the healthy control, DM and DR groups, respectively. A comparison of the mean abundance of the genera showed that the microbiomes in different cohorts differed from each other (Fig. 3A, B). In the DM group, one genus decreased and five genera increased in abundance (Table 2), and three genera decreased in abundance in the DR group compared with the healthy control (Table 3). Additionally, between the DM and DR groups, 24 genera decreased, and 2 genera increased in abundance in the DR group (Table 4). Box plots also showed a significant difference in abundance in genera in DM and DR compared with their respective controls (Fig. 4). Heat map analysis with the discriminatory genera indicated that the microbiomes of control, DM and DR formed three clusters. The microbiomes of DR formed a distinct cluster, whereas the microbiomes of healthy control overlapped with the DM and DR microbiomes (Fig. 5).
A Genera in the bacterial microbiomes from aqueous humour of individuals in control (HC, n = 4), diabetic (DM, n = 4) and diabetic retinopathy (DR, n = 4) groups. B Mean abundance of bacterial genera from aqueous humour microbiomes of individuals in control (HC, n = 4), diabetic (DM, n = 4) and diabetic retinopathy (DR, n = 4) groups
Box plots of genera exhibiting significant (P-value < 0.05) differential abundance in aqueous humour bacterial microbiomes of individuals in control (HC, n = 4), diabetic (DM, n = 4) and diabetic retinopathy (DR, n = 4) groups. Differentially abundant genera having a mean abundance of > 0.1% in at least one group of samples have been depicted. Median abundances (horizontal line) and interquartile ranges are indicated in the plots
Two-dimensional heat map representing rank normalized abundances (scaled between 0 and 1) of 15 differentially abundant bacterial genera (mean abundance of > 0.05% in at least one group) in the aqueous humour bacterial microbiomes of individuals in control (HC, n = 4), diabetic (DM, n = 4) and diabetic retinopathy (DR, n = 4) groups. The discriminating genera were arranged along the two dimensions (axes) on the basis of hierarchical clustering
Interactions Among the Bacterial Genera from Aqueous Humour of Individuals in Control, DM, and DR Groups
Interaction networks of microbiomes (HC, n = 4; DM, n = 4 and DR, n = 4) were generated on the basis of pair-wise correlations between abundances of different bacterial genera, and several hub genera were identified (exhibiting > 10 positive or negative or both interactions). A total of 18 hub taxa were unique to the healthy control group, 31 taxa were unique to DM and 11 taxa were unique to DR. Healthy control and DM groups shared two hub genera; four hub genera were common between DM and DR groups (Supplementary Table 2).
Dysbiosis in the Gut
Analysis of the gut microbiomes from the stool samples of 12 participants indicated a similar dysbiosis in DM and DR cohorts and a clear distinction among the healthy control, DM and DR groups (Fig. 6) as reported in our earlier study; hence, the results are not mentioned in detail in the current manuscript. A comparison of the microbiomes from the aqueous humour and the gut revealed several genera (> 1 mean abundance in any group) common to both aqueous humour and gut (Table 5).
Two-dimensional heat map representing rank normalized abundances (scaled between 0 and 1) of 18 significantly different genera (P-value ≥ 0.05) from the gut bacterial microbiomes of individuals in control (HC, n = 4), diabetic (DM, n = 4) and diabetic retinopathy (DR, n = 4) groups. The discriminating genera were arranged along the two dimensions (axes) on the basis of hierarchical clustering
Discussion
The preliminary analysis of the ocular and gut samples of our study participants indicates the following three distinct features:
-
a)
All eyes have an intraocular microbiome irrespective of the status of health.
-
b)
Aqueous humour dysbiosis is distinct from the gut microbiome, evident from the heatmap clustering (Figs. 5, 6).
-
c)
Aqueous humour of people with DR has unique genera relative to aqueous humour in people without DR or DM.
We are not the first to report the possibility of an intraocular microbiome. Human organs and their internal fluids are considered sterile in normal conditions. Using PCR, electron microscopy, culture techniques and sequencing in 1000 eyes, Deng et al. have reported the presence of microbiota in intraocular fluids of “normal” eyes; in their study, Propionibacterium spp. was detected in > 70% of eyes [10]. The authors noted disease-specific signals for age-related macular degeneration and glaucoma, just as we noted for DR in our study. Further, Deng et al. distinguished the intraocular microbiome from the blood and the ocular surface n using heat map analysis [10]. In our experiment, we assessed the relationship between the intraocular microbiome and the gut microbiome, considering that the gut may be an obvious source of the intraocular microbiota. However, we could not ascertain any significant differences between the gut and the ocular samples, which could reflect the relatively protean nature of the “exposed” gut microenvironment compared with its “protected” ocular counterpart. While our findings may also be due to specific properties of the ocular fluid, as in the case of DM or DR acting as enrichment media, intraocular microbiota may also have causative implications on DR, as has been considered by Deng et al. for glaucoma or macular degeneration [10]. Ours is a limited preliminary analysis, and it is too early to answer such questions. However, intraocular microbiota may prove to be a pathology-defining discovery for DR, like the earlier reports of intraocular microbiome (bacterial, fungal and viral) in the vitreous fluids of normal (control) and post-fever retinitis [1,2,3] from our group.
The gut microbiome is affected by many factors, such as age, diet, alcohol intake and medication [34]. Wilmanski et al., using a dissimilarity matrix on the Arivale cohort, noted that the gut microbiome begins to drift from middle life to a unique composition in people older than 80 years and that individuals are less healthy where such a drift does not occur [34]. Even the results from the ELDERMET cohort published a decade ago indicated the presence of unusual phyla in older individuals (> 65 years) compared with the very young ones (< 9 years) [6]. Other studies have also noted higher levels of gut alpha diversity in healthy centenarians and that a lack of personalized drifts indicates less 4-year survival [23, 34]. Deng et al. reported the presence of P. acnes or total bacterial DNA soon after births in the aqueous humour of rats, and it was absent in the eyes of unborn rats. Further, they also stated that the count stabilized into adulthood. The longitudinal rat intraocular microbiome changes appeared to mirror the human gut microbiome changes [10]. Considering the gut–retina axis, an end-organ dysbiosis is closer to the eye condition than other human micro-environments and may reflect long-term gut changes compared with short-term local volatilities in the gut. However, at present, our data on the intraocular microbiome (including ours) is only preliminary, and such a hypothesis should be considered appropriately [1,2,3].
A change in diet habits causes a temporary alteration in the gut microbiome profile. Studies have shown that self-reported diet measures have no or minimal effects on the gut microbiome [34]. Diet may explain nearly 20% of the gut microbiome in humans, but alterations in dietary habits do not influence this microbiome completely [24]. Short-term interventions may change the gut microbiome, but the core microbiome remains grossly stable [24]. A change in diet causes a temporary change in the gut microbiomes with reversion to its original state within days after the dietary intervention is discontinued [9]. In our study, we chose the UK Diabetes and Diet Questionnaire, validated for South Asia, to assess diet’s impact [12]. Our data showed apparent differences in the intraocular microbiome of DM and DR groups. Even though their dietary scores were similar, it indicates that diet has less impact on the intraocular microbiome. Other factors also impact the gut microbiome. Some of these could be heritability (up to 9%) [15] and circadian rhythm (up to 15%) [31]. We have not evaluated these factors in the current study; these should also be accounted for in future intraocular microbiome studies.
Tables 3 and 4 show the uniqueness of the intraocular microbiome of people with DM and DR compared with those with DM without DR and healthy controls. There was a gross reduction in the anti-inflammatory bacteria in the aqueous humour of people with DR. Incidentally, these observations are similar to our earlier ones with gut microbiome in people with DR but less obvious than the current study. In our earlier study on the gut microbiome, we noted a higher difference between controls and DM, and there was an overlap between DM and DR groups [7, 8, 32]. However, the current data on the intraocular microbiome show a wider separation across the DM and DR groups compared with the gut microbiome (Figs. 5, 6).
This study includes various strengths. These data are the first attempt to link the gut to the eye, thus bridging the gut–ocular or gut–retina axis. There are no precise relations between dysbiosis at the ocular and gut levels (Table 5). We set the abundance bars at 1% so that restricted, meaningful data could be analysed. We noted some similarities in abundance patterns of the gut and the eye (5 genera), but in most cases (16 genera), there was no relationship. Remarkably, three genera were restricted and appeared unique to either of the two micro-environments (two in the gut, one in the eye). The interaction hubs (Supplementary Table 2) add to this functional uniqueness of the intraocular microbiome.
This study also included weaknesses. Comparison of the three groups is limited, as they were different in terms of age, and age is known to affect the gut microbiome [34]. Our data are preliminary, with a small sample size (12 patients in three groups) and need further validation for consistency with larger studies and studies across different age groups to develop a longitudinal picture. A cohort model looking at repeated sampling may not be feasible as the eye does not remain naïve to microbes after an initial procedure, at least with the currently available methods of ocular sampling. We believe that the intraocular microbiome is likely to have a different relationship with DR compared with the gut microbiome, and the former may be more significant than the latter. Being likely “less volatile” and “untouched” than the gut, it could be a better therapeutic target to develop microbiome-related interventions for ocular diseases such as DR.
Conclusions
Our preliminary results show that an intraocular microbiome exists in health and disease. Furthermore, dysbiosis of the aqueous humour has distinctive features compared with the gut. Amongst these two micro-environments, the intraocular milieu may have more specific features in people with DR. The latter fact needs to be evaluated with an appropriate larger-sized study, and if proven consistent, will provide a promising therapeutic target to retard DR.
References
Arunasri K, Mahesh M, Sai Prashanthi G, Jayasudha R, Kalyana Chakravarthy S, Tyagi M, Pappuru RR, Shivaji S. Mycobiome changes in the vitreous of post fever retinitis patients. PLoS ONE. 2020;15: e0242138.
Arunasri K, Sai Prashanthi G, Tyagi M, Pappuru RR, Shivaji S. Intraocular viral communities associated with post-fever retinitis. Front Med. 2021;8:724195. https://doi.org/10.3389/fmed.2021.724195.
Arunasri K, Mahesh M, Sai Prashanthi G, Jayasudha R, Kalyana Chakravarthy S, Tyagi M, Pappuru RR, Shivaji S. Comparison of the vitreous fluid bacterial microbiomes between individuals with post fever retinitis and healthy controls. Microorganisms. 2020;8:751.
Beli E, Yan Y, Moldovan L, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. 2018;67:1867–79.
Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun A, Barnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41:963–70.
Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108:4586–91.
Das T, Jayasudha R, Chakravarthy S, Prashanthi GS, Bhargava A, Tyagi M, Rani PK, Pappuru RR, Sharma S, Shivaji S. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci Rep. 2021;11:2738.
Das T, Takkar B, Sivaprasad S, Thanksphon T, Taylor H, Wiedemann P, Nemeth J, Nayar PD, Rani PK, Khandekar R. Recently updated global diabetic retinopathy screening guidelines: commonalities, differences, and future possibilities. Eye (Lond). 2021;35:2685–98.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Deng Y, Ge X, Li Y, Zou B, Wen X, Chen W, Lu L, Zhang M, Zhang X, Li C, Zhao C. Identification of an intraocular microbiota. Cell discovery. 2021;7:1–2.
Emadian A, England CY, Thompson JL. Dietary intake and factors influencing eating behaviours in overweight and obese South Asian men living in the UK: mixed method study. BMJ Open. 2017;7: e016919.
England CY, Thompson JL, Jago R, Cooper AR, Andrews RC. Development of a brief, reliable and valid diet assessment tool for impaired glucose tolerance and diabetes: the UK Diabetes and Diet Questionnaire. Public Health Nutr. 2017;20:191–9.
Floyd JL, Grant MB. The gut-eye axis: lessons learned from murine models. Ophthalmol Ther. 2020;9:499–513.
GBD 2019 Diseases and Injuries Collaborators (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 17;396(10258):1204–1222.
Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, Bell JT, Clark AG, Ley RE. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19:731–43.
Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. https://doi.org/10.1016/j.ebiom.2019.11.051.
Huang Y, Wang Z, Ma H, Ji S, Chen Z, Cui Z, Chen J, Tang S. Dysbiosis and implication of the gut microbiota in diabetic retinopathy. Front Cell Infect Microbiol. 2021;11: 646348.
Jayasudha R, Chakravarthy SK, Prashanthi GS, et al. Alterations in gut bacterial and fungal microbiomes are associated with bacterial keratitis, an inflammatory disease of the human eye. J Biosci. 2018;43:835–56.
Jayasudha R, Das T, Kalyana Chakravarthy S, Sai Prashanthi G, Bhargava A, Tyagi M, Rani PK, Pappuru RR, Shivaji S. Gut mycobiomes are altered in people with type 2 diabetes mellitus and diabetic retinopathy. PLoS ONE. 2020;15: e0243077.
Kalyana Chakravarthy S, Jayasudha R, Ranjith K, et al. Alterations in the gut bacterial microbiome in fungal keratitis patients. PLoS ONE. 2018;13: e0199640.
Kalyana Chakravarthy S, Jayasudha R, Sai Prashanthi G, et al. Dysbiosis in the gut bacterial microbiome of patients with uveitis, an inflammatory disease of the eye. Indian J Microbiol. 2018;58:457–69.
Khan R, Sharma A, Ravikumar R, Parekh A, Srinivasan R, George RJ, Raman R. Association between gut microbial abundance and sight-threatening diabetic retinopathy. Invest Ophthalmol Vis Sci. 2021;62:19.
Kong F, Deng F, Li Y, Zhao J. Identification of gut microbiome signatures associated with longevity provides a promising modulation target for healthy aging. Gut Microbes. 2019;10:210–5.
Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. 2019;11:2862.
McKnight DT, Huerlimann R, Bower DS, Schwarzkopf L, Alford RA, Zenger KR. microDecon: A highly accurate read subtraction tool for the post-sequencing removal of contamination in metabarcoding studies. Environ DNA. 2019;1:14–25. https://doi.org/10.1002/edn3.11.
Padakandla SR, Das T, Sai Prashanthi G, Angadi KK, Reddy SS, Reddy GB, Shivaji S. Dysbiosis in the gut microbiome in streptozotocin-induced diabetes rats and follow-up during retinal changes. Invest Ophthalmol Vis Sci. 2021;62:31.
Padakandla SR, Das T, Sai Prashanthi G, Angadi KK, Reddy SS, Reddy GB, et al. Gut mycobiome dysbiosis in rats showing retinal changes indicative of diabetic retinopathy. PLoS ONE. 2022;17: e0267080. https://doi.org/10.1371/journal.pone.0267080.
Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem. 2019;63:101–8.
Shivaji S, Jayasudha R, Chakravarthy SK, et al. Alterations in the conjunctival surface bacterial microbiome in bacterial keratitis patients. Exp Eye Res. 2021;203: 108418.
Soni D, Sagar P, Takkar B. Diabetic retinal neurodegeneration as a form of diabetic retinopathy. Int Ophthalmol. 2021;41:3223–48. https://doi.org/10.1007/s10792-021-01864-4.
Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, Kuperman Y. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–29.
Thakur PS, Aggarwal D, Takkar B, Shivaji S, Das T. Evidence suggesting the role of gut dysbiosis in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2022;63:21.
Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513–21.
Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, Earls JC, Zimmer A, Glusman G, Robinson M, Yurkovich JT. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3:274–86.
World Health Organization. Regional Office for South-East Asia. (2020). Strengthening diagnosis and treatment of Diabetic Retinopathy in SEA Region. World Health Organization. Regional Office for South-East Asia. https://apps.who.int/iris/handle/10665/334224/. Accessed 17 October, 2022.
Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Acknowledgements
Funding
A) All authors: Hyderabad Eye Research Foundation, Hyderabad, India; B) Brijesh Takkar: DBT/Wellcome Trust India Alliance Clinical Research Centre Grant awarded to IHOPE (grant number IA/CRC/19/1/610010). No funding was received for the publication of this article.
Author Contributions
Taraprasad Das, Sisinthy Shivaji, Brijesh Takkar, Rajagopalaboopathi Jayasudha: concepts and review of literature. Brijesh Takkarand Rajagopalaboopathi Jayasudha: data collection. Rajagopalaboopathi Jayasudhaand Shalem Raj Padakandla: analysis of data. All authors: writing the manuscript and critical review. Taraprasad Das: obtaining funding.
Disclosures
All named authors confirm that they have no conflicts of interest to declare.
Prior Presentation
Data have been partially presented at India - ARVO in September 2022, at Hyderabad Retina Society meeting in 2022, and at Asia Pacific Vitreo-retina Society meeting in 2022.
Compliance with Ethics Guidelines
The study protocol was reviewed by the institutional review board, and ethical clearance was obtained (LEC-BHR-P-04–20-426). Informed consent was obtained before enrolment, and it adhered to the tenets of the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Das, T., Padakandla, S.R., Shivaji, S. et al. Intraocular Microbiome in Diabetes and Diabetic Retinopathy: A Pilot Study. Ophthalmol Ther 12, 1109–1126 (2023). https://doi.org/10.1007/s40123-023-00660-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00660-w