Abstract
Introduction
Intrapartum fever occurs frequently during labor. The purpose of this study was to investigate the effects of epidural dexmedetomidine on maternal temperature, pain score and adverse effects during labor analgesia.
Methods
A total of 600 full-term primiparous parturients were randomly divided into two groups. The dexmedetomidine group (Group Dex, n = 300) received 0.1% ropivacaine with 0.5 µg/mL dexmedetomidine for epidural analgesia during labor, while the control group (Group C, n = 300) received 0.1% ropivacaine alone. The maternal temperature, visual analogue scale (VAS) and Ramsay sedation score (RSS) were recorded, and the systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) were monitored. Side effects, if any, were also recorded.
Results
The incidence of intrapartum fever was lower in Group Dex than in Group C (4.1% vs. 8.7%, χ2 = 5.07, P = 0.024). VAS values from the time of 3 cm cervical dilatation to 10 cm cervical dilatation were also lower in Group Dex than in Group C (1.0 ± 0.9 vs. 1.3 ± 0.7, t = 3.62, P < 0.001; 2.8 ± 0.8 vs. 3.3 ± 0.8, t = 8.09, P < 0.001; 3.1 ± 0.9 vs. 3.3 ± 0.8, t = 3.88, P < 0.001; 3.6 ± 0.8 vs. 4.1 ± 1.0, t = 5.86, P < 0.001, respectively). HR from the time of 3 cm cervical dilatation to 10 cm cervical dilatation was lower during labor in Group Dex than in Group C (80.0 ± 4.3 vs. 83.1 ± 5.4 beats/min, t = 7.58, P < 0.001; 81.1 ± 4.0 vs. 83.7 ± 5.5 beats/min, t = 6.48, P < 0.001; 78.9 ± 5.4 vs. 81.5 ± 6.3 beats/min, t = 5.41, P < 0.001; 83.1 ± 5.3 vs. 84.8 ± 5.6 beats/min, t = 3.75, P < 0.001, respectively), while SBP and DBP were similar between the two groups. The incidence of adverse events during labor was also similar between the two groups.
Conclusion
The present study showed that dexmedetomidine could reduce the incidence of intrapartum fever and relieve pain during labor without increasing adverse events.
Trial Registration
ChiCTR-OPC-16008548.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The mechanisms of intrapartum fever are controversial. |
Dexmedetomidine increases analgesic effects. |
Dexmedetomidine may reduce the incidence of intrapartum fever. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13135880.
Introduction
The epidural technique is widely used for pain management during labor [1]. Intrapartum fever frequently occurs during labor, and women receiving epidural analgesia are at increased risk [2, 3]. Intrapartum fever is strongly associated with neonatal encephalopathy and cerebral palsy [4, 5]. However, the mechanisms of intrapartum fever are controversial. It is well known that anesthetics may inhibit the maternal temperature regulation center. Dexmedetomidine, an α2-adrenergic agonist, has been used successfully for epidural labor analgesia, with fewer side effects [6,7,8]. We hypothesized that dexmedetomidine could reduce the incidence of intrapartum fever during labor analgesia without increasing adverse events. The aim of this study was to investigate the effects of epidural dexmedetomidine on intrapartum fever, pain score and adverse effects during labor analgesia.
Methods
This study was approved by the Ethics Committee of Women and Children’s Hospital of Jiaxing University (reference number: 2016-036). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All participants provided informed consent to participate in the study. The trial was registered at www.chictr.org.cn (registration number: ChiCTR-OPC-16008548). A total of 600 full-term primiparous parturients were divided into two groups using a random number code. The dexmedetomidine group (Group Dex, n = 300) received 0.1% ropivacaine with 0.5 µg/ml dexmedetomidine for epidural analgesia, while the control group (Group C, n = 300) received 0.1% ropivacaine alone for epidural analgesia. Inclusion criteria included American Society of Anesthesiologists (ASA) grade I/II status, age 20–35 years, weight 50–85 kg, gestational week ≥ 37 weeks, and the ability of the subject, following assessment, to undergo vaginal delivery. Exclusion criteria included temperature from the ear canal > 37 °C, platelet count < 90 × 109/L, coagulation disorder, bradycardia, puncture point infection, serious cardiopulmonary disease, or liver or renal function disorder. The anesthetists, physicians and midwives were blinded to grouping.
The room temperature was set at 24 °C. Blood pressure (BP), heart rate (HR) and pulse oxygen saturation (SpO2) were monitored at 10-min intervals, and venous access was established. With parturients positioned in the left lateral position, the epidural technique was performed at level L2–3 using the air loss-of-resistance technique when cervical dilation was about 2 cm. An epidural catheter was advanced 3–5 cm cephalad into the epidural space and fixed. A 3 ml test dose of 1.5% lidocaine was administered through the epidural catheter to determine whether it had entered a blood vessel or the subarachnoid space, after which an initial volume of 10 ml analgesic solution was administered. The analgesic solutions consisted of 0.1% ropivacaine with 0.5 µg/ml dexmedetomidine (Jiangsu Hengrui Medicine Company, China) in Group Dex, and 0.1% ropivacaine in Group C, which were infused with a patient-controlled analgesia pump (Jiangsu Aipeng Medical Devices Co., Ltd, China). The parameters of the pump were set as follows: maintenance dose of 6 ml/h, a bolus of 6 ml and locked time of 15 min. The parturients were instructed to use the pump to inject a bolus dose to relieve pain. Five units of oxytocin were administered intravenously to increase contractions when progress was insufficient. Fetal heart rate was monitored continuously using an ultrasonic Doppler system (Oxford Company, UK).
The highest level of sensory block was measured within 30 min after administration using an alcohol cotton ball placed in the middle of the torso. The temperature from the ear canal (T) was measured by a thermometer (Kaz USA, Inc., PRO 4000, MA, USA). Maternal temperature and pain scores were recorded at 60-min intervals during the time from the beginning of epidural analgesia to 10 cm cervical dilatation. The highest temperature was noted during the time from the beginning of epidural analgesia to 10 cm cervical dilatation. The time between initiation of epidural analgesia to the highest temperature was also noted during labor. Intrapartum fever was defined as maternal temperature ≥ 38 °C. Acetaminophen 0.5 g was administered orally if maternal temperature increased to ≥ 39 °C. Ephedrine 6 mg was given intravenously when systolic blood pressure was < 80% of baseline. Supplemental oxygen was administered if SpO2 was below 95%. Pain intensity was assessed using a visual analogue scale (VAS) (0, no pain; 10, worst pain). Motor block was assessed using the Bromage score (0 = no motor loss, 1 = inability to flex hip, 2 = inability to flex hip and knee, 3 = inability to flex hip, knee and ankle). The incidence of intrapartum fever, hypotension, respiratory depression, bradycardia, excessive sedation nausea and vomiting was recorded. Hypotension was defined as systolic blood pressure (SBP) < 80% of baseline, respiratory depression was defined as SpO2 < 94% when inhaling air and respiratory rate < 10 breaths/min, and bradycardia was defined as HR < 60 beats/min. The level of sedation was evaluated every 60 min using the Ramsay sedation scale score (RSS: 1, patient anxious, agitated or restless; 2, patient cooperative, oriented and tranquil alert; 3, patient responds to commands; 4, asleep, but with brisk response to light glabellar tap or loud auditory stimulus; 5, asleep, sluggish response to light glabellar tap or loud auditory stimulus; 6, asleep, no response) [9]. Excessive sedation was defined as RSS of greater than 4. APGAR scores were recorded at 1 and 5 min after delivery.
Sample Size
The primary outcome was intrapartum maternal fever. Based on the incidence of intrapartum fever (7–10%) [4, 10], we calculated that a sample size of 250 women in each group would have 80% power to detect a difference of 4% in the incidence of intrapartum fever between the two groups with two-sided α error of 0.05. Allowing for dropouts, 300 subjects in each group were needed to enroll in the study.
Statistical Analysis
Statistical analysis was performed using the SPSS17.0 (IBM, NY, USA) software package. Numerical variables were presented as mean and standard deviation (SD) and analyzed by two-way analysis of variance for repeated measures. Intergroup comparison of parametric data was performed using a t test, and nonparametric data were analyzed by the Mann–Whitney U test. Categorical data were presented as numbers and analyzed using the chi-square test or Fisher’s exact test. The significance level was set at P < 0.05.
Results
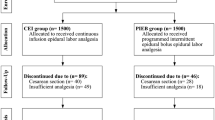
A total of 600 women were enrolled in the study. Five hundred and seventy-eight women completed the study, while 22 patients were excluded from the study due to a prolonged first labor stage (> 12–16 h) or to fetal distress in utero (1 case) or required cesarean section (Fig. 1).
The data for the women are shown in Table 1. There were no significant differences with respect to maternal age, height, weight, gestational weeks, labor type, highest level of sensory block or use of oxytocin between the two groups, but there were significant differences in terms of the first labor duration and time between initiation of epidural analgesia and the highest temperature in labor (358 ± 72 vs. 375 ± 84 min, t = 3.36, P = 0.01; 4.4 ± 0.6 vs. 4.7 ± 0.8 h, t = 5.09, P < 0.001, respectively), and the dose of analgesics was lower in Group Dex (51.4 ± 6.8 vs. 53.8 ± 7.6 ml, t = 4.01, P < 0.001).
SBP, diastolic blood pressure (DBP) and HR are shown in Fig. 2a–c. SBP and DBP were similar between the groups (P > 0.05), while HR during the time from 3 to 10 cm cervical dilatation after analgesia was lower in Group Dex than in Group C (80.0 ± 4.3 vs. 83.1 ± 5.4 beats/min, t = 7.58, P < 0.001; 81.1 ± 4.0 vs. 83.7 ± 5.5 beats/min, t = 6.48, P < 0.001; 78.9 ± 5.4 vs. 81.5 ± 6.3 beats/min, t = 5.41, P < 0.001; 83.1 ± 5.3 vs. 84.8 ± 5.6 beats/min, t = 3.75, P < 0.001, respectively).
a–c Comparison of SBP, DBP and HR in the two groups. SBP and DBP were similar between groups (P > 0.05), while HR at the time from 4 to 10 cm cervical dilatation after analgesia were lower in the dexmedetomidine group than in the control group (*P < 0.01). SBP systolic blood pressure, DBP diastolic blood pressure, HR heart rate
VAS scores from the time of 3 cm cervical dilatation to 10 cm cervical dilatation were lower in Group Dex than in Group C (Fig. 3) (1.0 ± 0.9 vs. 1.3 ± 0.7, t = 3.62, P < 0.001; 2.8 ± 0.8 vs. 3.3 ± 0.8, t = 8.09, P < 0.001; 3.1 ± 0.9 vs. 3.3 ± 0.8, t = 3.88, P < 0.001; 3.6 ± 0.8 vs. 4.1 ± 1.0, t = 5.86, P < 0.001, respectively).
The side effects are shown in Table 2. The incidence of intrapartum fever was lower in Group Dex (4.1% vs. 8.7%, χ2 = 5.07, P = 0.024). Maternal temperature was higher at 4 h after analgesia and 10 cm cervical dilatation in Group Dex compared to Group C (37.3 ± 0.4 vs. 37.5 ± 0.5 °C, t = 5.51, P < 0.001; 36.7 ± 0.4 vs. 36.9 ± 0.4 °C, t = 4.45, P < 0.001, respectively), while maternal temperature was similar at other time points during labor (Fig. 4). No excessive sedation (RSS > 4) was observed. The incidence of hypotension, respiratory depression, bradycardia, excessive sedation, nausea and vomiting was similar between groups.
Discussion
The present study found that dexmedetomidine could reduce the incidence of intrapartum fever and increase the analgesic effects of local anesthetics, without increasing adverse events.
Intrapartum fever likely has multiple causes. The infectious etiology of maternal fever includes chorioamnionitis and viral infections, among others. Noninfectious factors include high room temperature, administration of medications raising maternal temperature and the administration of neuraxial analgesia. Epidural analgesia leads to an increased incidence of intrapartum fever; however, the exact mechanism of maternal fever is unknown [3, 11]. The most current evidence supports the mechanistic involvement of noninfectious inflammation [12 ]. Intrapartum maternal fever is strongly associated with systemic and/or regional noninfectious inflammation, likely mediated by interleukin-6 [13, 14]. The mechanism of epidural-related maternal fever is complex and controversial. It may be related to an imbalance in heat and cold after epidural analgesia. Fusi et al. [15] posited that epidural block might inhibit heat dissipation, thus resulting in an increase in temperature during labor, while another study reported that epidural analgesia led to redistribution of heat from the core to the periphery, and thus loss of heat to the environment [16]. The exact mechanism of action for dexmedetomidine related to fever is unknown. Documents show that the α2-adrenergic receptor is associated with thermoregulation, and dexmedetomidine produces a reduction in body temperature through the α2 receptors [17, 18]. In this study, dexmedetomidine decreased the incidence of intrapartum maternal fever during labor. The possible reasons are as follows: Dexmedetomidine, an α2-adrenoceptor agonist, inhibited the maternal thermoregulation center by the central α2-adrenoceptor activity and reduced heat production. On the other hand, heat loss was increased with vasodilatation under epidural analgesia. In addition, dexmedetomidine inhibited tumor necrosis factor-α and interleukin-6 expression to alleviate inflammation by suppression of c-Jun N-terminal kinases [19]. A recent document reported that lower concentrations of ropivacaine reduced the rate of intrapartum fever during labor analgesia [20]. However, intrapartum fever in that study was defined as a body temperature of > 37.5 °C, whereas in our study it was defined as temperature of > 38.0 °C. Lange et al. [4] reported that intrapartum magnesium administration reduced the incidence of intrapartum fever during labor, as it inhibited the expression of interleukin-6. In our study, the highest temperature in labor occurred 4–8 h after analgesia, and a prolonged labor stage was associated with a higher risk of maternal fever, in concordance with previous research [4]. Douma et al. [21] reported that patient-controlled intravenous administration of remifentanil decreased the incidence of intrapartum maternal fever during labor compared with epidural analgesia. Camann et al. [22] suggested that maternal temperature was increased during labor using bupivacaine with or without fentanyl for epidural analgesia. Temperatures at 5 h and longer after analgesia were significantly greater than the pre-epidural temperatures, while maternal temperatures did not increase using only parenteral opioids for analgesia.
Local anesthetics combined with other analgesics can improve analgesic effects and reduce adverse events [23,24,25]. In our study, the incidence of hypotension, bradycardia, respiratory depression, excessive sedation, motor block nausea and vomiting was similar between groups, indicating that dexmedetomidine did not increase the incidence of side effects during labor. There were no significant differences in labor duration or delivery outcome between the two groups, and no adverse events were observed in the neonates. Epidural use of dexmedetomidine was safe for the mother and baby during labor.
The present study had several limitations. First, the exact mechanism of intrapartum fever is unknown. Second, other factors that lead to increases in body temperature may have influenced the results. Lastly, further research is needed to better understand the mechanism of intrapartum fever.
Conclusions
In conclusion, dexmedetomidine may reduce the incidence of intrapartum fever and increase analgesic effects during labor, without increasing adverse events.
References
Hawkins JL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362:1503–10.
Sharpe EE, Arendt KW. Epidural labor analgesia and maternal fever. Clin Obstet Gynecol. 2017;60:365–74.
Sultan P, David AL, Fernando R, Ackland GL. Inflammation and epidural-related maternal fever: proposed mechanisms. Anesth Analg. 2016a;122:1546–53.
Lange EMS, Segal S, Pancaro C, Wong CA, Grobman WA, Russell GB, et al. Association between intrapartum magnesium administration and the incidence of maternal fever: a retrospective cross-sectional study. Anesthesiology. 2017;127(6):942–52.
Greenwell EA, Wyshak G, Ringer SA, Johnson LC, Rivkin MJ. Lieberman E Intrapartum temperature elevation, epidural use, and adverse outcome in term infants. Pediatrics. 2012;129:e447–54.
Zhao Y, Xin Y, Liu Y, Yi X, Liu Y. Effect of epidural dexmedetomidine combined with ropivacaine in labor analgesia: a randomized double-blinded controlled study. Clin J Pain. 2017;33:319–24.
Zhang WP, Li C. EC50 of epidural ropivacaine combined with dexmedetomidine for labor analgesia. Clin J Pain. 2018a;35:905–8.
Wangping Z, Ming R. Optimal dose of epidural dexmedetomidine added to ropivacaine for epidural labor analgesia: a pilot study. Evid Based Complement Alternat Med. 2017;2017:7924148.
Zhang T, Yu Y, Zhang W, et al. Comparison of dexmedetomidine and sufentanil as adjuvants to local anesthetic for epidural labor analgesia:a randomized controlled trial. Drug Des Develop Ther. 2019;13:1171–5.
Towers CV, Yates A, Zite N, Smith C, Chernicky L, Howard B. Incidence of fever in labor and risk of neonatal sepsis. Am J Obstet Gynecol. 2017;216(6):596.
Sultan P, David AL, Fernando R, Ackland GL. Inflammation and epidural-related maternal fever: proposed mechanisms. Anesth Analg. 2016b;122:1546–53.
Arendt KW, Segal BS. The association between epidural labor analgesia and maternal fever. Clin Perinatol. 2013;40(3):385–98.
Segal S, Pancaro C, Bonney I, Marchand JE. Noninfectious fever in the near-term pregnant rat induces fetal brain inflammation: A model for the consequences of epidural-associated maternal fever. Anesth Analg. 2017;125(6):2134–40.
Goetzl L, Evans T, Rivers J, Suresh MS, Lieberman E. Elevated maternal and fetal serum interleukin-6 levels are associated with epidural fever. Am J Obstet Gynecol. 2002;187:834–8.
Fusi L, Maresh MJA, Steer PJ, Beard RW. Maternal pyrexia associated with the use of epidural analgesia in labour. Lancet. 1989;1:1250–2.
Holdcroft A, Hall GW, Cooper G. Redistribution of body heat during anaesthesia. Anaesthesia. 1979;34:758–64.
Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, et al. Assessment of the role of alpha2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–44.
Paris A, Ohlendorf C, Marquardt M, Bein B, Sonner JM, Scholz J, et al. Effect of meperidine on thermoregulation in mice: involvement of alpha2-adrenoceptors. Anesth Analg. 2005;100:102–6.
Zhang X, Wang J, Qian W, Zhao J, Sun L, Qian Y, et al. Dexmedetomidine inhibits tumor necrosis factor-alpha and interleukin 6 in lipopolysaccharide-stimulated astrocytes by suppression of c-Jun N-terminal kinases. Inflammation. 2014;37(3):942–9.
Zhou X, Li J, Deng S, Xu Z, Liu Z. Ropivacaine at different concentrations on intrapartum fever, IL-6 and TNF-α in parturient with epidural labor analgesia. Exp Ther Med. 2019;17:1631–6.
Douma MR, Stienstra R, Middeldorp JM, Arbous MS, Dahan A. Differences in maternal temperature during labour with remifentanil patient-controlled analgesia or epidural analgesia: a randomised controlled trial. Int J Obstet Anesth. 2015;24(4):313–22.
Camann WR, Hortvet LA, Hughes N, Bader AM, Datta S. Maternal temperature regulation during extradural analgesia for labour. Br J Anaesth. 1991;67(5):565–8.
Landau R, Schiffer E, Morales M, et al. The dose-sparing effect of clonidine added to ropivacaine for labor epidural analgesia. Anesth Analg. 2002;95:728–34.
Halpern SH, Carvalho B. Patient-controlled epidural analgesia for labor. Anesth Analg. 2009;108:921–8.
Wang X, Xu S, Qin X, et al. Comparison between the use of ropivacaine alone and ropivacaine with sufentanil in epidural labor analgesia. Medicine (Baltimore). 2015;94:e1882.
Acknowledgements
The authors would like to thank the midwives for collecting data in this study.
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s rapid service fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Study design: Z.W. Data collection: Z.Y and Z.W. Data analysis: Z.Y. Writing of manuscript: Z.W and L.L.
Disclosures
Li Li, Yang Zeyong and Zhang Wangping have nothing to disclose.
Compliance With Ethics Guidelines
This study was approved by the Ethics Committee of Women and Children’s Hospital of Jiaxing University (Reference number: 2016-036). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All participants consented to participate in this study.
Data Availability
The data will be accessible 6 months after publication. The documents will be available at http://www.qq.com, by contacting the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, L., Yang, Z. & Zhang, W. Epidural Dexmedetomidine for Prevention of Intrapartum Fever During Labor Analgesia: A Randomized Controlled Trial. Pain Ther 10, 391–400 (2021). https://doi.org/10.1007/s40122-020-00215-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-020-00215-y