Abstract
Introduction
The release of pro-inflammatory cytokines in critically ill patients with sepsis leads to endothelial dysfunction resulting in cardiocirculatory insufficiency. Their extracorporeal elimination using the cytokine adsorber CytoSorb® (CS) (adsorption of especially hydrophobic molecules < 60 kDa) might be promising, but data about the adsorption capacity as well as a potential harmful adsorption of anti-inflammatory cytokines are missing so far.
Methods
The prospective Cyto-SOLVE-study included 15 patients with sepsis or other hyperinflammatory conditions (interleukin 6 > 500 pg/ml), continuous kidney replacement therapy, and the application of CS. Various cytokines and chemokines were measured pre- and post-CS as well as in patients’ blood at predefined timepoints. Significant changes in the concentrations were detected with the Wilcoxon test with associated samples. Clearance of the adsorber (ml/min) was calculated with: \(blood\,flow*\frac{{concentration\, \left( {pre - post} \right)}}{{concentration\, \left( {pre} \right)}}.\)
Results
Most of the inflammatory mediators showed a high initial extracorporeal clearance of 70–100 ml/min after CS installation, which dropped quickly to 10-30 ml/min after 6 h of treatment. No difference in clearance was observed between pro- and anti-inflammatory cytokines. Despite extracorporeal adsorption, a significant (p < 0.05) decrease in the blood concentration after 6 h was only observed for the pro-inflammatory cytokines tumor necrosis factorα (TNF-α) (median 284 vs. 230 pg/ml), vascular endothelial growth factor (VEGF) (median 294 vs. 252 pg/ml), macrophage inflammatory protein 1a (MIP-1a) (median 11.1 vs. 9.0 pg/ml), and regulated upon activation, normal T cell expressed and secreted (RANTES) (median 811 vs. 487 pg/ml) as well as the anti-inflammatory cytokines interleukin 4 (median 9.3 vs. 6.4 pg/ml), interleukin 10 (median 88 vs. 56 pg/ml), and platelet-derived growth factor (PDGF) (median 177 vs. 104 pg/ml). A significant (p < 0.05) decrease in patients’ blood after 12 h was only detected for interleukin 10.
Conclusions
CS can adsorb pro- as well as anti-inflammatory mediators with no relevant difference regarding the adsorption rate. A fast saturation of the adsorber resulted in a rapid decrease of the clearance. The potential clinical benefit or harm of this unspecific cytokine adsorption needs to be evaluated in the future.
Trial Registration
ClinicalTrials.gov NCT04913298, registration date June 4, 2021.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Hyperinflammation, which is often observed in patients with sepsis and septic shock, leads to vasoplegia, capillary leakage, and circulatory insufficiency resulting in the clinical picture of shock. |
Cytokines play a key role in the development of shock, so their extracorporeal elimination might, in addition to treating the cause of shock, be useful. |
The question of interest was, which cytokines are adsorbed by the adsorber CytoSorb® and is there a saturation kinetic during application? |
What was learned from the study? |
The CytoSorb® adsorber can eliminate pro- as well as anti-inflammatory cytokines; however, the clearance of the different cytokines dropped to 10–30 ml/min after 6 h not leading to any relevant change in patients’ blood. |
As the unselective and low adsorption of different cytokines did not lead to a change in patients’ blood, routine clinical use cannot be recommended in this indication. |
Introduction
Critical hyperinflammatory conditions such as sepsis are associated with a high mortality rate in critically ill patients [2, 4]. The underlying disease can lead to an excessive immune response with the release of particularly pro-inflammatory cytokines [5, 14]. The cytokines in turn lead via signaling cascades to endothelial dysfunction with the development of a capillary leak. On the one hand, this leads to an increase in fluid in the interstitium with the risk of secondary pulmonary edema but also to hemodynamic instability due to intravascular hypovolemia and vasoplegia [29].
Regardless of the entity of the cytokine release, the main aim is to eliminate the cause as quickly as possible, e.g., by administering anti-infectives or surgical sanitation of the infection source [6]. In addition, the administration of volume and vasopressors is essential for maintaining adequate perfusion pressure [16]. Hydrocortisone is often administered as a supportive treatment to dampen the immune response [11].
In addition to the aforementioned interventions, adsorption procedures are another possible treatment option for patients with hyperinflammation [12]. The aim of these procedures is to achieve faster hemodynamic stabilization with improved outcome through the adsorption of cytokines. The underlying hypothesis is the restoration of homeostasis [12]. However, current guidelines (e.g., surviving sepsis campaign) do not give a recommendation for its routine use. One such method is the cytokine adsorber CytoSorb® (CS), which can adsorb especially hydrophobic molecules with a size of < 60 kDa [23]. The cartridge is often integrated into an existing kidney replacement circuit. The evidence for its use in sepsis and other hyperinflammatory conditions is still unclear [31]. Apart from various case reports and studies without a control group, even small randomized studies could not provide a benefit referring patients’ outcome.
Stockmann et al. observed no difference in inflammatory parameters in 50 patients admitted to the intensive care unit (ICU) with severe COVID-19 infection and treated with or without CS application [30]. This was also the result of Supady et al., although mortality was significantly higher in the group treated with CS [32]. During cardiac surgery, interleukin 8 (IL-8) and tumor necrosis factor alpha (TNF-α) were lower at the end of the operation in patients treated with CS integrated into the cardiopulmonary bypass than without CS; however, the effect was only short-lived and no longer detectable after 24 h [7]. Last, Schädler et al. observed a significant interleukin 6 (IL-6) removal in patients with sepsis by CS, however the plasma concentration was equal to patients with standard care [26]. However, only the pleiotropic cytokine IL-6 was investigated. These data demonstrate that there is often no difference in the comparison of cytokine concentrations in the blood between patients treated with CS and non-treated ones.
To date, the real in vivo adsorption performance of the cartridge in critically ill patients is neither known for pro- nor anti-inflammatory immunomodulating proteins. Furthermore, it has not been investigated whether the cartridge is saturated during the course of therapy and thus a loss of effectiveness occurs. The question of interest was therefore to evaluate the adsorption performance and saturation kinetics of CS for various pro- and anti-inflammatory modulators in patients treated at the intensive care unit with hyperinflammatory conditions.
Methods
Study Setting
The Cyto-SOLVE study was a monocentric, prospective trial investigating the adsorption rate and saturation kinetics of CS in three trial arms liver dysfunction, rhabdomyolysis, and hyperinflammation. The adsorption capacity for different pro- and anti-inflammatory cytokines in patients with sepsis or other hyperinflammatory conditions was investigated in the trial arm “hyperinflammation”. The study was registered at ClinicalTrials.gov on June 4, 2021 (NCT04913298). Patients were included between May 2021 and April 2023 during their stay at two ICUs at the LMU hospital in Munich.
Ethical Approval
Ethical approval was obtained from the ethical review committee of the Ludwig-Maximilians-Universität (registration number 21-236). Written informed consent was obtained from the patients or their legal representatives in line with the vote of the review board prior to study inclusion. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Study Population
The study included adult patients (≥ 18 years) with the necessity of continuous kidney replacement therapy (CKRT) due to an acute kidney injury (AKI) stage 2 or 3 diagnosed by the AKI classification of the KDIGO consensus criteria [1]. The indication of starting CKRT, the modality (continuous veno-venous hemodialysis (CVVHD) or continuous veno-venous hemodiafiltration (CVVHDF)), and the indication for using CS was at the responsibility of the attending physician. Twenty patients were included in each of the study arms, rhabdomyolysis [8] and liver dysfunction [9]. For inclusion in the hyperinflammation study arm, IL-6 concentration in patients’ blood had to be > 500 pg/ml prior to CS application. Exclusion criteria were no consent to the participation in the study and prior CS application. Seventeen patients were initially included in the hyperinflammation arm; two patients were excluded due to clotting of the extracorporeal circuit in the first 3 h (see Fig. 1).
Blood Sampling
CS was installed after the dialyzer into the CKRT circuit with the hemofilter Ultraflux AV 1000S (Fresenius Medical Care, Bad Homburg, Germany). Blood samples (EDTA tubes) were taken at the extracorporeal circuit directly before the cartridge (= pre-CS) and directly after the cartridge (= post-CS) at prespecified time points (10 min after starting CS treatment as well as 1, 3, 3, and 12 h after initiation). The different mediators were also measured in the arterial blood shortly before initiation of CS, after 6, and after 12 h. EDTA anticoagulated plasma was obtained by centrifugation of whole blood at the ICU right away after sampling. Separated plasma samples were immediately frozen and stored stably at − 80 °C until cytokine measurement within 6 months.
Data Collection
For data evaluation, demographic data as well as clinical and laboratory variables were collected from the electronic laboratory and patient information system. As part of daily clinical routine, various laboratory tests on automated clinical chemistry analyzers (Roche Diagnostics, Mannheim, Germany) were requested directly before the initiation of CS treatment.
Laboratory Measurements
A total of 19 different cytokines, chemokines and other modulators were quantified with Bio-Plex human cytokine multiplex assays (Bio-Rrad, Feldkirchen, Germany). Table 1 displays the different modulators and their role in hyperinflammatory conditions (pro- vs. - anti-inflammatory).
Statistical Analysis
Statistical analysis was performed with IBM SPSS statistics (Version 29.0. IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean with standard deviation (SD) (if there was a normal distribution) or median with interquartile range (IQR). T test with associated samples (if there was a normal distribution) or Wilcoxon-test with associated samples was used to compare the concentrations pre- and post-CS and the changes in the blood. Relative change (RC) was calculated with: \(\frac{{concentration \left( {post - pre} \right)}}{{concentration \left( {pre} \right)}}*100.\)
Clearance of the adsorber (ml/min) was calculated with: \(blood\,flow \left( {\frac{ml}{{min}}} \right)*\frac{{concentration\, \left( {pre - post} \right)}}{{concentration \,\left( {pre} \right)}}. \)
Results
Demographic and Clinical Data
In total, 15 patients were included in the evaluation. All patients had a hyperinflammatory condition with an IL-6 concentration > 500 pg/ml at the initiation of therapy. The intended duration of CS therapy was 12 h, which was attained in 11 patients. The extracorporeal circuit clotted in two patients between 6 and 12 h and two patients deceased between 6 and 12 h of therapy, so the last data point after 12 h is missing in those four patients. The mean age was 57 years and 80% were male. The mean Simplified Acute Physiology Score II (SAPS II) on the day of the CS treatment was 69 points and the 28-day mortality rate was 73.3%. The reasons for the hyperinflammatory condition were in descending order: intestinal ischemia (26.7%), acute respiratory distress syndrome, septic shock or compartment syndrome (each 20%), and pancreatitis or necrotizing fasciitis (6.7% each). All patients needed vasopressor support, whereby no significant change in dosage was observed during the application of CS. All patients were treated with anti-infective therapy, 80% with hydrocortisone, and 60% underwent surgical infection control. Detailed patient characteristics and laboratory parameters measured immediately before initiation of CS can be found in Table 2.
Clearance of Different Pro-inflammatory Modulators
A total of 13 particularly pro-inflammatory modulators were measured. It was not possible to obtain a quantitative result for each single parameters in all patients because the measurement results fell below the lower limit of quantification. The number of analyzed patients with complete measurement series is shown for each of the parameters (i.e., n = 9). Wilcoxon test with associated samples was used in the absence of a normal distribution. Supplemental Tables S1–S19 present detailed results of the measured mediators in each patient.
Interleukins
A significant (p < 0.02) extracorporeal IL-1β reduction (n = 9) was observed in the first 6 h of treatment. Furthermore, a significant extracorporeal reduction was also observed for IL-6 (n = 15, p < 0.03) and for IL-8 (n = 15, p < 0.01) in the first 3 h of application. As the IL-2 measurement was only possible in four patients, a statistical analysis was not rational. The median (IQR) clearance (ml/min) of the three interleukins IL-1β, IL-6, and IL-8 was 10 min after initiation 114 (77–125), 78 (74–123), and 91 (84–164) ml/min, and decreased significantly (p < 0.01) to 37 (16–42), 14 (9–18), and 33 (24–43) after 6 h, respectively.
IFN-γ, TNF-α, and VEGF
There was a significant (p < 0.01) extracorporeal reduction of IFN-γ (n = 12) in the first 6 h of treatment, of VEGF (n = 15, p < 0.02) in the first 3 h of treatment, and of TNF-α in the first 10 min of treatment (n = 15, p = 0.015). The median (IQR) clearance (ml/min) of IFN-γ, TNF-α, and VEGF was 10 min after initiation 84 (57 – 113), 30 (− 12 to 77), and 83 (65 to 92) ml/min, and decreased significantly (p < 0.01) to 24 (6 to 36), 6 (− 44 to 18), and 13 (− 13 to 45) after 6 h, respectively.
Chemokines
A significant extracorporeal reduction of interferon gamma-induced protein (IP-10) (n = 15, p < 0.01) and monocyte chemoattractant protein-1 (MCP-1) (n = 15, p < 0.01) was detected at all timepoints; macrophage inflammatory proteins 1a (MIP-1a) (n = 15, p < 0.01) was only significantly adsorbed in the first 6 h and normal T cell expressed and secreted (RANTES) (n = 15, p = 0.047) in the first hour of treatment. The median (IQR) clearance (ml/min) of IP-10, MCP-1, MIP-1a, and RANTES was 10 min after initiation 100 (98–212), 72 (71–94), 96 (89–183), and 43 (− 30 to 81) ml/min, and decreased to 87 (78–103), 19 (13–26), 37 (29–46), and − 29 (− 62 to 17) after 6 h, respectively (significant decrease for IP-10 and MCP-1 (p < 0.01)). Higher RANTES concentrations were observed post-CS compared to pre-CS from 1 h of application, resulting in a negative clearance.
Soluble Cell Adhesion Molecules
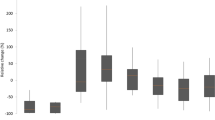
There was a significant extracorporeal reduction of intercellular adhesion molecule (ICAM) (n = 15, p < 0.04) as well as vascular cell adhesion molecule (VCAM) (n = 15, p < 0.05) in the first 6 h. The median (IQR) clearance (ml/min) of ICAM and VCAM was 10 min after initiation 30 (15–43) and 22 (15–37) ml/min, and decreased to 9 (2–11) and 11 (1–15) after 6 h, respectively (significant decrease p < 0.01 for ICAM). Figure 2 illustrates the median clearance of all pro-inflammatory mediators at the defined timepoints.
Median clearance of the adsorber for different pro-inflammatory mediators. IL interleukin, IFN interferon, TNF tumor necrosis factor, VEGF vascular endothelial growth factor, IP-10 interferon gamma-induced protein 10, MCP-1 monocyte chemoattractant protein-1, MIP-1 macrophage inflammatory protein-1, RANTES regulated upon activation, normal T cell expressed and secreted, ICAM intercellular adhesion molecule, VCAM vascular cell adhesion molecule
Clearance of Different Anti-inflammatory Mediators
Interleukins
There was a significant extracorporeal IL-4 (n = 11, p < 0.02) and IL-5 (n = 14, p < 0.05) reduction in the first 6 h of treatment as well as IL-10 (n = 14, p < 0.01) reduction in the total 12 h of treatment. The median (IQR) clearance (ml/min) of IL-4, IL-5, and IL-10 was 10 min after initiation 74 (63–115), 86 (66–99), and 102 (78–151) ml/min, and decreased significantly (p < 0.01) to 26 (22–43), 11 (0–41), and 32 (26–45) after 6 h, respectively.
Growth Factors
A significant extracorporeal reduction of fibroblast growth factor (FGF-basic) (n = 9, p < 0.04) was observed in the first 6 h and of platelet-derived growth factor (PDGF) (n = 14, p < 0.01) after 10 min of treatment. As a sufficient measurement of granulocyte/macrophage colony-stimulating factor (GM-CSF) was only possible in four patients, no statistical analysis was performed. The median (IQR) clearance (ml/min) of FGF-basic and PDGF was 10 min after initiation 70 (55–95) and 87 (65–132) ml/min, and decreased significantly (p < 0.01) to 34 (19–39) and 5 (− 39 to 25) after 6 h, respectively. Figure 3 illustrates the median clearance of all anti-inflammatory mediators at the defined timepoints.
Change in Patients’ Blood
Table 3 displays the median relative change after 6 and 12 h of treatment of the different mediators in patients’ blood.
Figure 4 illustrates the relative change of key pro- and anti-inflammatory mediators during CS application in patients’ blood.
Discussion
It is undisputed that causal therapy (e.g., anti-infectives, surgical infection control) has the highest priority and is the focus in patients with hyperinflammation of various origins [20]. Supportive therapy, such as the administration of volume and vasopressors, is also of great importance [34]. The idea that the extracorporeal removal of pathogenic substances (e.g., pro-inflammatory cytokines and endotoxins) can lead to a more rapid improvement in the patient's condition seems promising and has been used for over 10 years [13]. The immunological changes in patients with hyperinflammatory conditions are complex and the role of pro- and anti-inflammatory cytokines has not yet been conclusively clarified [14]. The significance of an unselective procedure that removes a previously unknown quantity of various substances remains unclear [24].
CS, which now has several CE marks, such as for the removal of myoglobin, was initially approved in 2011 for the elimination of cytokines in patients with hyperinflammation [23]. To date, a large number of studies have been published attributing clinical improvement (without control group) in patients achieved through causal, supportive, and additive therapy primarily to the use of CS [10, 21]. However, from the authors' point of view, these studies do not provide any evidence as lacking a control group. In addition, to date, primarily a change in cytokines in the blood has been investigated without knowing the actual contribution of CS to the change and without considering the adsorption of potentially beneficial parameters [22].
In our study, we measured many pro- and anti-inflammatory cytokines both in blood taken from the patient and in the extracorporeal circuit pre- and post-adsorber in critically ill patients with relevant hyperinflammation to assess the real in vivo adsorption capacity. There was not only a significant extracorporeal elimination of pro-inflammatory but also of anti-inflammatory cytokines. A drop in clearance after 3–6 h indicates increasing saturation of the adsorber. A significant decrease in the blood 6 h after the implementation of CS could only be detected for a total of four pro- (TNF-α, VEGF, MIP-1a, RANTES) and three anti-inflammatory cytokines (IL-4, IL-10, PDGF). In the case of RANTES, the clearance by CS was even negative, so the drop in blood was not caused by the device. At the end of the treatment interval (after 12 h), only the anti-inflammatory cytokine IL-10 was significantly lower compared to the concentration shortly before the start of CS treatment and the need for vasopressors remained the same over the therapy interval.
Back in 2015, Linden et al. determined in a pig model that extracorporeal adsorption does not automatically lead to a change in cytokines in the blood [18]. Furthermore, most cytokines have a short half-life of a few minutes [33]. Therefore, if the cause of cytokine release is adequately treated or eliminated, the cytokine concentration is expected to drop rapidly. Conversely, the question arises how much a cytokine clearance of i.e., 30–50 ml/min will contribute, if there is a sustained release. It must also be borne in mind that the adsorption process is non-selective. It has been shown that meropenem is not adsorbed by CS [17], but there is relevant vancomycin and linezolid adsorption with the risk of underdosing [15, 27]. In vitro adsorption of various antimycotics has also been demonstrated [28]. The use of an additive procedure without sufficient evidence could therefore result in a worse overall outcome by compromising causal therapies (e.g., with anti-infectives).
There are currently various debates about the value of adsorption procedures in sepsis or hyperinflammation. While a meta-analysis concludes that there is no positive effect of CS and refrains from using it [3], a consensus paper endorses its use in patients with hyperinflammation [19]. This is just one example of the extent to which personal opinions, rather than evidence-based recommendations, differ. From the authors' point of view, this work is all the more important, as it provides the first data on the real adsorption capacity in vivo of various pro- and anti-inflammatory cytokines after more than 10 years of routine use. It will hopefully provide the basis for a rational use of these procedures in sepsis, as recently promoted by Ronco et al. [25].
Finally, this study has several limitations. As the study focused on the elimination kinetics with extracorporeal measurement of different substances in vivo, no statement can be made on the change in patients’ outcome by the use of CS. This requires a confirmatory multicenter study with a relevant patient-centered endpoint. Furthermore, both CVVHD and CVVHDF were used as dialysis modalities; however, as all modulators were directly measured pre- and post-CS, this should not have any influence on the adsorption kinetics. Moreover, we cannot exclude whether a higher blood flow leads to an even faster saturation of the adsorber. The inclusion of 15 patients seems small at first, but sufficient to answer the question with regard to the adsorption of cytokines. Last, some of the measured parameters were below the lower limit of quantification in the blood in some of the patients. This is typical when many cytokines are measured, but has little influence for answering the primary question.
Conclusions
Our study was able to show that the use of CS results in significant adsorption of both pro- and anti-inflammatory cytokines in patients with hyperinflammation. The clearance (and therefore the adsorption capacity) decreases rapidly over time, indicating saturation of the adsorber. Due to the non-selective adsorption, it remains unclear whether this has a predominant benefit for the patient or poses a risk. Future randomized controlled studies should investigate patient-centered endpoints in order to assess the real benefit or harm of the device.
Data Availability
All data generated during this study are included in this article or as supplementary files.
References
Andrassy KM. Comments on “KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease.” Kidney Int. 2013;84:622–3.
Bala M, Catena F, Kashuk J, et al. Acute mesenteric ischemia: updated guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2022;17:54.
Becker S, Lang H, Vollmer Barbosa C, et al. Efficacy of CytoSorb(R): a systematic review and meta-analysis. Crit Care. 2023;27:215.
Cecconi M, Evans L, Levy M, et al. Sepsis and septic shock. Lancet. 2018;392:75–87.
Cuschieri J, Bulger E, Schaeffer V, et al. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 2010;34:346–51.
Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247.
Garau I, Marz A, Sehner S, et al. Hemadsorption during cardiopulmonary bypass reduces interleukin 8 and tumor necrosis factor alpha serum levels in cardiac surgery: a randomized controlled trial. Minerva Anestesiol. 2019;85:715–23.
Graf H, Grafe C, Bruegel M, et al. Myoglobin adsorption and saturation kinetics of the cytokine adsorber CytoSorb(R) in patients with severe rhabdomyolysis: a prospective trial. Ann Intensive Care. 2024;14:96.
Greimel A, Habler K, Grafe C, et al. Extracorporeal adsorption of protective and toxic bile acids and bilirubin in patients with cholestatic liver dysfunction: a prospective study. Ann Intensive Care. 2023;13:110.
Hawchar F, Tomescu D, Trager K, et al. Hemoadsorption in the critically ill-final results of the International CytoSorb Registry. PLoS ONE. 2022;17: e0274315.
Heming N, Sivanandamoorthy S, Meng P, et al. Immune effects of corticosteroids in sepsis. Front Immunol. 2018;9:1736.
Honore PM, Hoste E, Molnar Z, et al. Cytokine removal in human septic shock: where are we and where are we going? Ann Intensive Care. 2019;9:56.
Honore PM, Jacobs R, Joannes-Boyau O, et al. Newly designed CRRT membranes for sepsis and SIRS–a pragmatic approach for bedside intensivists summarizing the more recent advances: a systematic structured review. ASAIO J. 2013;59:99–106.
Jarczak D, Nierhaus A. Cytokine storm-definition, causes, and implications. Int J Mol Sci. 2022;23:11740.
Kohler T, Schwier E, Kirchner C, et al. Hemoadsorption with CytoSorb((R)) and the early course of linezolid plasma concentration during septic shock. J Artif Organs. 2022;25:86–90.
Lat I, Coopersmith CM, De Backer D, et al. The surviving sepsis campaign: fluid resuscitation and vasopressor therapy research priorities in adult patients. Intensive Care Med Exp. 2021;9:10.
Liebchen U, Scharf C, Zoller M, et al. No clinically relevant removal of meropenem by cytokine adsorber CytoSorb((R)) in critically ill patients with sepsis or septic shock. Intensive Care Med. 2021;47:1332–3.
Linden K, Scaravilli V, Kreyer SF, et al. Evaluation of the CytoSorb hemoadsorptive column in a pig model of severe smoke and burn injury. Shock. 2015;44:487–95.
Mitzner S, Kogelmann K, Ince C, et al. Adjunctive hemoadsorption therapy with CytoSorb in patients with septic/vasoplegic shock: a best practice consensus statement. J Clin Med. 2023;12:7199.
Niederman MS, Baron RM, Bouadma L, et al. Initial antimicrobial management of sepsis. Crit Care. 2021;25:307.
Persic V, Jerman A, Malgaj Vrecko M, et al. Effect of CytoSorb coupled with hemodialysis on interleukin-6 and hemodynamic parameters in patients with systemic inflammatory response syndrome: a retrospective cohort study. J Clin Med. 2022;11:7500.
Poli EC, Alberio L, Bauer-Doerries A, et al. Cytokine clearance with CytoSorb(R) during cardiac surgery: a pilot randomized controlled trial. Crit Care. 2019;23:108.
Poli EC, Rimmele T, Schneider AG. Hemoadsorption with CytoSorb((R)). Intensive Care Med. 2019;45:236–9.
Ronco C, Bellomo R. Hemoperfusion: technical aspects and state of the art. Crit Care. 2022;26:135.
Ronco C, Chawla L, Husain-Syed F, et al. Rationale for sequential extracorporeal therapy (SET) in sepsis. Crit Care. 2023;27:50.
Schadler D, Pausch C, Heise D, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: a randomized controlled trial. PLoS ONE. 2017;12: e0187015.
Scharf C, Weinelt F, Schroeder I, et al. Does the cytokine adsorber CytoSorb((R)) reduce vancomycin exposure in critically ill patients with sepsis or septic shock? A prospective observational study. Ann Intensive Care. 2022;12:44.
Schneider AG, Andre P, Scheier J, et al. Pharmacokinetics of anti-infective agents during CytoSorb hemoadsorption. Sci Rep. 2021;11:10493.
Siddall E, Khatri M, Radhakrishnan J. Capillary leak syndrome: etiologies, pathophysiology, and management. Kidney Int. 2017;92:37–46.
Stockmann H, Thelen P, Stroben F, et al. CytoSorb rescue for COVID-19 patients with vasoplegic shock and multiple organ failure: a prospective, open-label, randomized controlled pilot study. Crit Care Med. 2022;50:964–76.
Supady A, Brodie D, Wengenmayer T. Extracorporeal haemoadsorption: does the evidence support its routine use in critical care? Lancet Respir Med. 2022;10:307–12.
Supady A, Weber E, Rieder M, et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med. 2021;9:755–62.
Volante E, Moretti S, Pisani F, et al. Early diagnosis of bacterial infection in the neonate. J Matern Fetal Neonatal Med. 2004;16(Suppl 2):13–6.
Zampieri FG, Bagshaw SM, Semler MW. Fluid therapy for critically ill adults with sepsis: a review. JAMA. 2023;329:1967–80.
Acknowledgements
We thank Antonia Greimel, Nils Maciuga, and Clara I. Brozat for their contribution in the collection of blood samples. We thank the patients and their legal representatives for participating in this study.
Funding
This project was funded by the Else Kröner-Fresenius-Stiftung (2021_EKEA.101). The journal’s Rapid Service Fee was also funded by the Else Kröner-Fresenius-Stiftung.
Author information
Authors and Affiliations
Contributions
Christina Scharf, Mathias Bruegel, Uwe Liebchen, and Michael Zoller: concept and design. Christina Scharf, Helen Graf, and Caroline Gräfe: drafting the manuscript. Aljoscha Wegener and Vassilissa Wustrow: revising the manuscript. Michael Paal, Wolfgang Wilfert, and Felix L. Happich data analysis. All authors participated in the manuscript writing process and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
Christina Scharf got speaker fees from CytoSorbents Europe GmbH. Michael Zoller received consulting honoraries from CytoSorbents Europe GmbH. Uwe Liebchen received consulting honoraries from CytoSorbents Europe GmbH and Medows and was part of an advisory board of Roche Diagnostics International Ltd. Helen Graf, Caroline Gräfe, Mathias Bruegel, Felix L. Happich, Vassilissa Wustrow, Aljoscha Wegener, Wolfgang Wilfert, and Michael Paal have nothing to disclose.
Ethical Approval
Ethical approval was obtained from the ethical review committee of the Ludwig-Maximilians-Universität (registration number 21-236). Written informed consent was obtained from the patients or their legal representatives in line with the vote of the review board prior to study inclusion. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Graf, H., Gräfe, C., Bruegel, M. et al. Extracorporeal Elimination of Pro- and Anti-inflammatory Modulators by the Cytokine Adsorber CytoSorb® in Patients with Hyperinflammation: A Prospective Study. Infect Dis Ther 13, 2089–2101 (2024). https://doi.org/10.1007/s40121-024-01028-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-01028-8