Abstract
Introduction
Cytokine adsorption using the CytoSorb® adsorber has been proposed in various clinical settings including sepsis, ARDS, hyperinflammatory syndromes, cardiac surgery or recovery after cardiac arrest. The aim of this analysis is to provide evidence for the efficacy of the CytoSorb® adsorber with regard to mortality in various settings.
Methods
We searched PubMed, Cochrane Library database and the database provided by Cytosorbents™ (01.1.2010–29.5.2022). We considered randomized controlled trials and observational studies with control groups. The longest reported mortality was defined as the primary endpoint. We computed risk ratios and 95%-confidence intervals and used DerSimonian and Lairds random effects model. We analysed all studies combined and divided them into the subgroups: sepsis, cardiopulmonary bypass surgery (CPB), other severe illness, SARS-CoV-2 infection and recovery from cardiac arrest. The meta-analysis was registered in advance (PROSPERO: CRD42022290334).
Results
Of an initial 1295 publications, 34 studies were found eligible, including 1297 patients treated with CytoSorb® and 1314 controls. Cytosorb® intervention did not lower mortality (RR [95%-CI]: all studies 1.07 [0.88; 1.31], sepsis 0.98 [0.74; 1.31], CPB surgery 0.91 [0.64; 1.29], severe illness 0.95 [0.59; 1.55], SARS-CoV-2 1.58 [0.50; 4.94]). In patients with cardiac arrest, we found a significant survival advantage of the untreated controls (1.22 [1.02; 1.46]). We did not find significant differences in ICU length of stay, lactate levels, or IL-6 levels after treatment. Of the eligible 34 studies only 12 were randomized controlled trials. All observational studies showed moderate to serious risk of bias.
Interpretation
To date, there is no evidence for a positive effect of the CytoSorb® adsorber on mortality across a variety of diagnoses that justifies its widespread use in intensive care medicine.
Similar content being viewed by others
Introduction
Massive release of cytokines into the bloodstream is the pathophysiological culprit of many life-threating diseases. Pro-inflammatory cytokines lead to vasodilation, capillary leakage, and coagulopathy. Anti-inflammatory cytokines can cause relative immunosuppression leading to secondary nosocomial infections. The uncontrolled release of both types of cytokines has the potential to end in multiple organ failure [1].
Various blood purification techniques, such as dialysis using high cut-off membranes, hemoadsorption, high volume hemofiltration, and plasma exchange have been proposed to unselectively reduce cytokine levels [2]. CytoSorb® is one of the most widely used blood purification devices, which can reduce the level of hydrophobic molecules with a molecular mass up to 55 kDa. [3] Thus, the device adsorbs cytokines, bile acids, and myoglobin. CytoSorb® is in clinical use in patients with an excessive immune response such as in sepsis, ARDS, SARS-CoV-2 infections, hyperinflammatory syndromes, and during and after cardiac surgery using cardiopulmonary bypass (CPB). In addition, CytoSorb® may be useful in liver failure, elimination of DOACs or certain acute intoxications [4]. The device is considered to be safe and well-tolerated. However, there is no consensus on the effectiveness.
The aim of this systematic review and meta-analysis is to evaluate the impact of CytoSorb® in all previously described medical conditions. The primary endpoint is the longest reported mortality. Furthermore, ICU length of stay, norepinephrine requirements, IL-6 and lactate levels will be compared.
Methods
We performed a systematic search of the PubMed and Cochrane Library database. We used “CytoSorb” as the keyword in all fields. Related articles were evaluated for additional publications. In addition, the database provided by CytoSorbents (https://literature.cytosorb-therapy.com/?_ga=2.58770730.642024760.1646225438-27175153.1645687922) was screened. The last research update was conducted on May 29 2022.
We considered randomized controlled trials (RCT) and observational studies comprising a control group. Case reports were excluded. Studies had to contain information on the primary endpoint. If the results of multiple studies referred to the same patients, the larger study was considered. The intervention group had to receive at least one treatment with the CytoSorb® adsorber. The control group was allowed to differ only by the CytoSorb® treatment. Intervention and control groups each had to include at least three patients. There was no language restriction. Studies were selected by two investigators independently; in case of disagreement, consensus was sought. In accordance with the recommendations of the Bias Method Group of the Cochrane network, the quality of the studies was assessed using the ROBINS-I tool (Risk of Bias in Non-randomized Studies of Interventions) or the RoB 2 tool (revised tool for Risk of Bias in randomized trials). The data were collected by two investigators independently and summarized in an Excel file. If graphs were provided instead of exact values, we analysed the data using “WebPlotDigitizer” (https://apps.automeris.io/wpd/).
The primary endpoint was the longest reported mortality (30-day, in-hospital or ICU mortality). If more than one value was given, the value with the longest observational period was chosen. The primary endpoint was computed as relative risk. Subgroup analyses of the primary endpoint were performed for different medical conditions (sepsis, CPB surgery, severe illness, SARS-CoV-2 and cardiac arrest). Subgroup analyses for different study designs were also performed (randomized controlled trials vs. observational studies with and without propensity score matched controls). The random effect model (DerSimonian and Laird) was used for inference.
Secondary endpoints were ICU length of stay and hospital length of stay. We extracted information on norepinephrine dose, mean arterial pressure (MAP), CRP, PCT, lactate and interleukin-6 (IL-6) levels before and after CytoSorb® intervention. The levels of inflammatory markers after CytoSorb® intervention were defined as the first reported value after the start of CytoSorb® treatment. In addition, we evaluated the SOFA, SAPS-2, and APACHE-2 scores to compare the control and intervention groups. Also, we screened all studies for reported adverse events. Secondary endpoints were reported as risk differences (treatment group—control group) for all studies combined and for the individual subgroups. Analyses of secondary end points were performed if at least two studies reported relevant data. For elective cardiopulmonary bypass surgery, sepsis scores and physiologic endpoints were not applicable. The original studies reported secondary endpoints most often as mean or median values. We followed the recommendations of McGrath et al [5]: assuming normal distribution, mean values were considered to be equal to medians. Medians reported with interquartile ranges or minimum and maximum values were converted to mean values and standard deviation using the method of Luo et al. [6] Subsequently, the effect of mean values was assessed using a random effect model with the DerSimonian-Laird method. Effects of median values were calculated by the quantile estimation method. Information on secondary endpoints is subsequently always reported first as mean and then as median.
Trial sequential analysis (TSA) was performed using the software provided by the Copenhagen Trial Unit on https://ctu.dk. We performed TSA of RCTs in the subgroup of CPB only, as this subgroup had the highest number of studies. We used the following settings for the analysis: RRR to detect 10%, Power 20%, p < 0.05 two-sided, α-spending function: O’Brien Flemming; the mortality in the control group was 14.85 [7].
Risk of bias was assessed with the tools ROBINS-I and RoB 2 by two investigators independently. We used funnel plots to assess publication bias and applied the GRADE methodology to assess certainty. We used R 4.1.0 with the packages meta, metafor, metamedian, lqmm, hmisc, estmeansd, forestplot and writexl for all analyses. P values less than 0.05 were considered to be statistically significant. Heterogeneity was assessed using I2 and Cochrane’s q. The study protocol was registered on the PROSPERO database (registration number: CRD42022290334).
Results
Study search
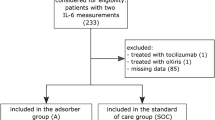
The search returned 1295 hits, of which 249 were excluded due to duplication. One thousand and forty-six titles were screened. Of 381 reports, first the abstracts and then the full texts were reviewed for eligibility. Six studies were excluded due to overlapping study participants [8,9,10,11,12,13]. Another eight studies did not provide information on our defined primary endpoint [14,15,16,17,18,19,20,21]. One study was excluded because the group size was too small (n = 2), and three studies were excluded due to the lack of standard therapy in the control group [3, 22,23,24]. Thus, 34 studies were available for analysis (Fig. 1). No paediatric patients were included since all studies involving children were either case reports or did not have a control group.
Study characteristics
Eight studies investigated the effect of CytoSorb® in patients with sepsis or septic shock [25,26,27,28,29,30,31,32]. Fifteen studies used it in cardiopulmonary bypass surgery [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Four studies employed CytoSorb® in patients suffering from SARS-CoV-2 [48,49,50,51]. Another three studies used CytoSorb® in patients after cardiovascular arrest [52,53,54]. There were four other studies that investigated the effect of CytoSorb® yet could not be assigned to any of the previously mentioned groups. We defined this group as “severe illness” and included the following conditions: severely injured polytrauma patients, patients with ARDS requiring veno-venous-ECMO, severely ill patients with an IL-6 ≥ 10,000 pg/ml and patients with severe acute pancreatitis [55,56,57,58]. There were twelve randomized controlled trials (RCT), twelve observational studies in which intervention and control groups were matched by propensity scores (PSM), and ten observational studies with otherwise selected controls (nPSM) (Table 1). A total of 1297 patients were treated with CytoSorb® and compared to 1314 patients without CytoSorb®.
Mortality
The longest reported mortality was not significantly different between Cytosorb® and control groups for all studies combined (RR 1.07 [0.88; 1.31]) and in the subgroups sepsis (RR 0.98 [0.74; 1.31]), CPB surgery (RR 0.91 [0.64; 1.29]), severe illness (RR 0.95 [0.59; 1.55]), and SARS-CoV-2 (RR 1.58 [0.50; 4.94]). In patients with cardiac arrest, we found a significant survival advantage of the untreated controls (RR 1.22 [1.02; 1.46]) (Fig. 2). The results were very similar if ICU mortality, in-hospital mortality or 30-day mortality were assessed (Additional file 1: Figures S1–S3).
Subgroup analysis by study design showed that in none of the subgroups irrespective of the disease state examined and the quality of the study a significant difference in survival could be established. Comparing the pooled analysis across all disease states, one could get the impression the results get worse with higher quality of study design. (Fig. 3).
In some subgroups, we found high levels of heterogeneity. RCTs had significantly less between-study variance (I2 = 0%; Q-statistic p = 0.452) than observational studies (PSM I2 = 59.5%; Q-statistic p = 0.004; nPSM I2 = 77.51%; Q-statistic p < 0.001) (Figs. 2, 3).
Trail sequential analysis
TSA was performed in the subgroup of CBP only for the primary endpoint. Figure 4 shows that with actual 478 patients the necessary number of 2801 is not reached. However, the cumulative Z-Score is already in the area of futility, suggesting that additional studies will not change the result.
ICU and hospital length of stay
The ICU length of stay was not significantly different between CytoSorb® and control groups, neither for all studies combined, nor for subgroups of different diagnoses. The differences between groups (treatment group—control group) were compared first as mean values (± SD) and second as median values [and IQR] (Table 2).
Norepinephrine and mean arterial pressure (MAP)
There was no significant difference in norepinephrine dose (ug/kg/min) or MAP at baseline. For patients after cardiac arrest, the control group required a significantly higher norepinephrine dose when comparing mean values (− 0.16 (± 0.15)). This observation could not be confirmed when comparing medians (− 0.09 (± 0.26)). All other subgroups showed no significant difference between CytoSorb® and control groups in norepinephrine dose after the start of treatment. Furthermore, the blood pressure (MAP) 24 h after the start of treatment showed no significant difference (Table 3).
Baseline SOFA, SAPS-2, and APACHE-2
None of these scores showed significant differences between CytoSorb® and control groups at baseline (Additional file 1: Table S1).
CRP, PCT, lactate and IL-6 levels
The CRP levels were not significantly different at baseline or at first measurement after treatment (Additional file 1: Table S2).
PCT levels were significantly higher in the control group for patients with sepsis when considering median values (– 7.60 (± 7.76) / – 7.13 [– 13.29; – 0.97]). PCT levels in all studies combined and in patients with cardiac arrest were not significantly different. Furthermore, there were no significant differences in PCT levels after treatment (Additional file 1: Table S2).
There were no significant differences in lactate levels at baseline or at first measurement after treatment begin for any subgroup (Additional file 1: Table S3).
Solely the subgroup “severe disease” had significantly higher median IL-6 levels in the intervention group, the mean values showed no significant difference. After treatment there was also no difference in IL-6 levels between CytoSorb® and control groups in any subgroup (Additional file 1: Table S3).
Adverse events
The occurrence of adverse events was monitored in 22 of the 34 studies. However, most studies did not clearly define what was considered an adverse event. Schädler et al. and Gleason et al. reported a decline in platelet count that they saw in connection with the CytoSorb® absorber [32, 40]. Other studies also observed a decline in platelet count that was found to be unrelated to the specific use of the CytoSorb® adsorber and more likely due to contact with an extracorporeal membrane in general [35, 36, 47, 52, 54]. None of the studies concluded that CytoSorb® is hazardous.
Risk of bias
The risk of bias was assessed with the tools ROBINS-I and RoB 2 and publication bias by funnel plots. We applied the GRADE methodology to assess certainty. Some studies showed a considerable risk of bias (Additional file 1: Figs. S4 and S5). Using the ROBINS-I tool, all nPSM studies were rated as "serious risk”, since they did not measure or control for confounding factors. In addition, the funnel plot shows evidence of publication bias in nPSM studies with studies showing negative results remaining unpublished (Additional file 1: Fig. 6). In contrast, the PSM studies did control for confounding, so that most PSM studies were rated "moderate risk". However, some studies excluded certain confounders that we considered relevant (Nemeth et al. 2018 excluded the three significant baseline characteristics (Seattle Heart Failure Score, IMPACT score, and high urgent status) from their matching [39]; Rieder et al. [52] did not consider the RESP score and PRESERVE score in the matching). Overall, the funnel plot shows no evidence of publication bias in PSM studies (Additional file 1: Fig. S7). The randomized controlled trials raised a few concerns as well. In general, the assessment of risk of bias was performed very strictly. Studies that did not follow the intention-to-treat protocol were rated as "serious risk". Likewise, studies with drop-outs were rated as "serious risk". If a parameter mentioned in the study protocol was changed or not analysed without justification, this also resulted in a rating of "serious risk". However, we are convinced that the overall quality of the RCTs is good and that there is no publication bias (Additional file 1: Fig. S8).
Discussion
This meta-analysis comprises data of 34 studies, including a total of 1297 patients treated with CytoSorb® compared to 1314 controls, in order to evaluate the use of CytoSorb® absorbers. We did not observe a significant reduction in mortality due to CytoSorb® intervention.
The use of blood purification methods in the treatment of severely-ill patients suffering from infection, sepsis and multiple organ failure has been investigated for over 30 years. [59] In a cytokine storm the massive release of cytokines can cause a severe inflammatory syndrome that leads to organ dysfunction [60]. Thus, the removal of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α from the blood stream should curb the hyperactivation of the immune system and reduce the systemic response. From this hypothesis, varying justifications for hemoadsorption arise. The removal of a single targeted cytokine was unsuccessful in the past, so that efforts were concentrated on the unselective removal of cytokines via blood purification methods such as by hemoperfusion using adsorber systems such as CytoSorb® [61, 62]. The use of CytoSorb® and other adsorbers is widespread, although to-date there is no consensus on the effectiveness of treatment. [63, 64]
One limitation of our meta-analysis is the heterogeneity of the studies. The observations of individual studies differed substantially, which we attribute to various reasons. First, there are a fair number of studies with only few study participants yet large effect sizes. Eight studies included less than 15 patients per group [26, 28, 39, 45, 46, 51, 56, 58]. These studies achieved great effects with CytoSorb® intervention. By contrast, there are larger RCTs and observational studies with propensity score matching (PSM) with notably smaller effect sizes. Secondly, the use of CytoSorb® differed in many ways. The number of adsorbers, duration of therapy, time from diagnosis to first use of the adsorber, and blood flow rate were very inconsistent. Thirdly, medical conditions were compared that differ completely in their pathophysiology. For this reason, we included the subgroup analysis of different medical conditions. Finally, the variances proved to be quite large due to the non-normal distribution of some variables. We overcame this limitation by performing subgroup analyses. We found no positive effect of CytoSorb® in any of the examined subgroups. This is true for the subgroups of different medical conditions as well as for the subgroup of different types of study design.
Not only is the positive effect of CytoSorb® intervention in terms of mortality reduction uncertain, even studies investigating the theoretical rationale behind treatment, namely the reduction in blood cytokine levels, show divergent results. The CytoSorb® adsorber has been shown to remove IL-1β, IL-6, IL-8, and TNF α in vivo, ex vivo and in vitro studies [65, 66]. Other studies have shown no significant reduction in IL-6 levels [32, 50]. A possible explanation provided by Honore et al. for stable cytokine levels despite removal during hemoabsorption is the shift of further cytokines from the interstitium into the bloodstream [67]. Furthermore cytokine release is continuous, so that a selective removal for a few hours a day may not be sufficient to have an impact on treatment success. Both are possible reasons why perhaps CytoSorb® failed to reduce mortality. Also it has been argued that some of the disease states, in which CytoSorb® is used, mainly SARS-CoV-2 and cardiopulmonary bypass surgery are not accompanied by particularly high cytokine levels [32, 50]. Assuming an adequate removal of pro-inflammatory cytokines, a further explanation for the failure to reduce mortality with Cytosorb® is the unselective removal of all hydrophobic plasma components ranging up to molecular mass of 55 kDa. This means that anti-inflammatory mediators, hormones and clotting factors are also removed [68]. In addition, antibiotics are also removed by the Cytosorb® adsorber, which may cause subtherapeutic antibiotic levels, impacting the success of sepsis treatment and overall survival [69].
We urgently need evidence from randomized controlled trials for the various medical conditions in which CytoSorb® is used taking into account the dynamics of the different diseases. At the moment only the use in CPB might be sufficiently assessable as shown by the trial sequential analysis.
Conclusion
In conclusion, there is no evidence of a reduction in mortality by treatment with CytoSorb® in any of the examined conditions. Therefore, we cannot recommend the use of CytoSorb® in intensive care patients unless clear evidence is generated. We need adequately designed RCTs in specific medical conditions targeting the right patients. Hence, ground work needs to be undertaken to identify patients likely to respond to the therapy, e.g. very high cytokine levels, and the optimal time point for therapy of the respective condition.
Availability of data and materials
As the meta-analysis is based on published data only, all data are publicly available.
References
Chakraborty RK, Burns B. Systemic inflammatory response syndrome. In: StatPearls. StatPearls Publishing; 2022. Accessed March 7, 2022. http://www.ncbi.nlm.nih.gov/books/NBK547669/
Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: a meta-analysis of randomized trials*. Crit Care Med. 2013;41(9):2209–20. https://doi.org/10.1097/CCM.0b013e31828cf412.
Scharf C, Liebchen U, Paal M, Irlbeck M, Zoller M, Schroeder I. Blood purification with a cytokine adsorber for the elimination of myoglobin in critically ill patients with severe rhabdomyolysis. Crit Care. 2021;25(1):41. https://doi.org/10.1186/s13054-021-03468-x.
Köhler T, Schwier E, Praxenthaler J, Kirchner C, Henzler D, Eickmeyer C. Therapeutic modulation of the host defense by hemoadsorption with CytoSorb®—basics, indications and perspectives—a scoping review. Int J Mol Sci. 2021;22(23):12786. https://doi.org/10.3390/ijms222312786.
McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biom J. 2020;62(1):69–98. https://doi.org/10.1002/bimj.201900036.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. https://doi.org/10.1177/0962280216669183.
Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C (2017). User Manual for Trial Sequential Analysis (TSA) [Pdf]. 2nd Ed. Copenhagen: Copenhagen Trial Unit, pp. 1–119. Downloadable from Ctu.Dk/Tsa. Accessed on: 05.03.2023 Online.
Poli E. Effect of cytoadsorbant device on post-cardio-pulmonary bypass inflammation in ESICM LIVES 2018: Effect of cytoadsorbant device on post-cardio-pulmonary bypass inflammation in ESICM LIVES 2018. Intensive Care Med Exp. 2018;6(S2):40. https://doi.org/10.1186/s40635-018-0201-6.
László I, Von Seth M, Hillered L, et al. Effects of adsorption of cytokines early in septic shock (the ‘ACESS-trial’)—results of the pilot study in 37th International Symposium on Intensive Care and Emergency Medicine (part 3 of 3): Brussels, Belgium. 21–24 March 2017. Crit Care. 2017;21(S1):58. https://doi.org/10.1186/s13054-017-1629-x.
Brouwer WP, Duran S, Ince C. Improved survival beyond 28 days up to 1 year after CytoSorb treatment for refractory septic shock: a propensity-weighted retrospective survival analysis. Blood Purif. 2021;50(4–5):539–45. https://doi.org/10.1159/000512309.
Woitsch S. Verbesserung der intrakraniellen Sauerstoffversorgung während Operationen im tiefen hypothermen Kreislaufstillstand mit Hämoadsorption. 2018;(Der Hauptstadtkongress der DGAI für Anästhesiologie und Intensivtherapie mit Pfl egesymposium und Rettungsdienstforum).
Schädler D, Porzelius C, Jörres A, et al. A multicenter randomized controlled study of an extracorporeal cytokine hemoadsorption device in septic patients. Crit Care. 2013;17(S2):P62. https://doi.org/10.1186/cc12000.
Schittek G. Beeinflussung des intensivstationären Therapieverlaufs durch die Einführung der Absorptionstherapie bei Patienten mit septischen Schock und akuten Nierenversagen. Published online 2018.
Born, F. Systemic Inflammatory Response Syndrome in der Herzchirurgie_Kardiotechnik 02–2014 - English Translation.pdf. Published online February 2014. http://armaghansalamat.com/media/brands/Cytosorbents/Literature/2014_Born%20F%20et%20al.,%20Systemic%20Inflammatory%20Response%20Syndrome%20in%20der%20Herzchirurgie_Kardiotechnik%2002-2014%20-%20English%20Translation.pdf
Gliga S, Orth HM, Lübke N, Timm J, Luedde T, Jensen BEO. Multicentric Castleman’s disease in HIV patients: a single-center cohort diagnosed from 2008 to 2018. Infection. 2021;49(5):945–51. https://doi.org/10.1007/s15010-021-01618-5.
Mehta Y, Singh A, Singh A, Gupta A, Bhan A. Modulating the inflammatory response with hemadsorption (CytoSorb) in patients undergoing major aortic surgery. J Cardiothorac Vasc Anesth. 2021;35(2):673–5. https://doi.org/10.1053/j.jvca.2020.06.028.
Lebreton G, Dorgham K, Quentric P, Combes A, Gorochov G, Schmidt M. Longitudinal cytokine profiling in patients with severe COVID-19 on extracorporeal membrane oxygenation and hemoadsorption. Am J Respir Crit Care Med. 2021;203(11):1433–5. https://doi.org/10.1164/rccm.202011-4140LE.
Lewis TC, Merchan C, Toy B, et al. Impact of CytoSorb hemoadsorption on sedation requirements in patients with severe COVID-19 on venovenous extracorporeal membrane oxygenation. ASAIO J. 2021;67(8):856–61. https://doi.org/10.1097/MAT.0000000000001513.
Garau I, März A, Sehner S, et al. Hemadsorption during cardiopulmonary bypass reduces interleukin 8 and tumor necrosis factor α serum levels in cardiac surgery: a randomized controlled trial. Minerva Anestesiol. 2019. https://doi.org/10.23736/S0375-9393.18.12898-7.
Bok S. CytoSorb: what is the effect on the circulation? in ESICM LIVES 2017: 30th ESICM Annual Congress September 23–27, 2017. Intensive Care Med Exp. 2017;5(S2):44. https://doi.org/10.1186/s40635-017-0151-4.
Laubach CM. Cytokine and DAMP adsorption in septic acute kidney injury in ESICM LIVES 2018: Paris, France. 20-24 October 2018. Intensive Care Med Exp. 2018;6(S2):40. https://doi.org/10.1186/s40635-018-0201-6.
Bottari G, Lorenzetti G, Severini F, Cappoli A, Cecchetti C, Guzzo I. Role of hemoperfusion with CytoSorb associated with continuous kidney replacement therapy on renal outcome in critically III children with septic shock. Front Pediatr. 2021;9:718049. https://doi.org/10.3389/fped.2021.718049.
Berlot G, Pintacuda S, Moro E, et al. Effects of tocilizumab versus hemoadsorption combined with tocilizumab in patients with SARS-CoV-2 pneumonia: preliminary results. Int J Artif Organs. 2022;45(1):75–80. https://doi.org/10.1177/0391398821989334.
Bottari G, Murciano M, Merli P, et al. Hemoperfusion with CytoSorb to manage multiorgan dysfunction in the spectrum of hemophagocytic lymphohistiocytosis syndrome in critically Ill children. Blood Purif. 2022;51(5):417–24. https://doi.org/10.1159/000517471.
Schittek GA, Zoidl P, Eichinger M, et al. Adsorption therapy in critically ill with septic shock and acute kidney injury: a retrospective and prospective cohort study. Ann Intensive Care. 2020;10(1):154. https://doi.org/10.1186/s13613-020-00772-7.
Akil A, Ziegeler S, Reichelt J, et al. Combined use of CytoSorb and ECMO in patients with severe pneumogenic sepsis. Thorac Cardiovasc Surg. 2021;69(03):246–51. https://doi.org/10.1055/s-0040-1708479.
Wendel Garcia PD, Hilty MP, Held U, Kleinert EM, Maggiorini M. Cytokine adsorption in severe, refractory septic shock. Intensive Care Med. 2021;47(11):1334–6. https://doi.org/10.1007/s00134-021-06512-0.
Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. J Crit Care. 2019;49:172–8. https://doi.org/10.1016/j.jcrc.2018.11.003.
Kogelmann K, Hübner T, Schwameis F, Drüner M, Scheller M, Jarczak D. First evaluation of a new dynamic scoring system intended to support prescription of adjuvant CytoSorb hemoadsorption therapy in patients with septic shock. J Clin Med. 2021;10(13):2939. https://doi.org/10.3390/jcm10132939.
Rugg C, Klose R, Hornung R, et al. Hemoadsorption with CytoSorb in septic shock reduces catecholamine requirements and in-hospital mortality: a single-center retrospective ‘Genetic’ matched analysis. Biomedicines. 2020;8(12):539. https://doi.org/10.3390/biomedicines8120539.
Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care. 2019;23(1):317. https://doi.org/10.1186/s13054-019-2588-1.
Schädler D, Pausch C, Heise D, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. Eller K, ed. PLOS ONE. 2017;12(10):e0187015. https://doi.org/10.1371/journal.pone.0187015
Poli EC, Alberio L, Bauer-Doerries A, et al. Cytokine clearance with CytoSorb® during cardiac surgery: a pilot randomized controlled trial. Crit Care. 2019;23(1):108. https://doi.org/10.1186/s13054-019-2399-4.
Hassan K, Kannmacher J, Wohlmuth P, Budde U, Schmoeckel M, Geidel S. Cytosorb adsorption during emergency cardiac operations in patients at high risk of bleeding. Ann Thorac Surg. 2019;108(1):45–51. https://doi.org/10.1016/j.athoracsur.2018.12.032.
Bernardi MH, Rinoesl H, Dragosits K, et al. Effect of hemoadsorption during cardiopulmonary bypass surgery—a blinded, randomized, controlled pilot study using a novel adsorbent. Crit Care. 2016;20(1):96. https://doi.org/10.1186/s13054-016-1270-0.
Taleska Stupica G, Sostaric M, Bozhinovska M, et al. Extracorporeal hemadsorption versus glucocorticoids during cardiopulmonary bypass: a prospective, randomized. Controlled Trial Cardiovasc Ther. 2020;2020:1–15. https://doi.org/10.1155/2020/7834173.
Saller T, Hagl C, Woitsch S, et al. Haemadsorption improves intraoperative haemodynamics and metabolic changes during aortic surgery with hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2019;56(4):731–7. https://doi.org/10.1093/ejcts/ezz074.
Santer D, Miazza J, Koechlin L, et al. Hemoadsorption during cardiopulmonary bypass in patients with endocarditis undergoing valve surgery: a retrospective single-center study. J Clin Med. 2021;10(4):564. https://doi.org/10.3390/jcm10040564.
Hassan K, Brüning T, Caspary M, et al. Hemoadsorption of rivaroxaban and ticagrelor during acute type A aortic dissection operations. Ann Thorac Cardiovasc Surg. 2022. https://doi.org/10.5761/atcs.oa.21-00154.
Gleason TG, Argenziano M, Bavaria JE, et al. Hemoadsorption to reduce plasma-free hemoglobin during cardiac surgery: results of REFRESH I pilot study. Semin Thorac Cardiovasc Surg. 2019;31(4):783–93. https://doi.org/10.1053/j.semtcvs.2019.05.006.
Träger K, Skrabal C, Fischer G, et al. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass—a case series. Int J Artif Organs. 2017;40(5):240–9. https://doi.org/10.5301/ijao.5000583.
Nemeth E, Kovacs E, Racz K, et al. Impact of intraoperative cytokine adsorption on outcome of patients undergoing orthotopic heart transplantation-an observational study. Clin Transplant. 2018;32(4):e13211. https://doi.org/10.1111/ctr.13211.
Zhigalov K, Van den Eynde J, Zubarevich A, et al. Initial experience with CytoSorb therapy in patients receiving left ventricular assist devices. Artif Organs. 2022;46(1):95–105. https://doi.org/10.1111/aor.14099.
Haidari Z, Wendt D, Thielmann M, et al. Intraoperative hemoadsorption in patients with native mitral valve infective endocarditis. Ann Thorac Surg. 2020;110(3):890–6. https://doi.org/10.1016/j.athoracsur.2019.12.067.
Wagner R, Soucek P, Ondrasek J, et al. Plasma levels of myocardial MicroRNA-133a increase by intraoperative cytokine hemoadsorption in the complex cardiovascular operation. J Clin Med Res. 2019;11(12):789–97. https://doi.org/10.14740/jocmr3989.
Asch S, Kaufmann TP, Walter M, et al. The effect of perioperative hemadsorption in patients operated for acute infective endocarditis—a randomized controlled study. Artif Organs. 2021;45(11):1328–37. https://doi.org/10.1111/aor.14019.
Diab M, Lehmann T, Bothe W, et al. Cytokine hemoadsorption during cardiac surgery versus standard surgical care for infective endocarditis (REMOVE): results from a multicenter randomized controlled trial. Circulation. 2022;145(13):959–68. https://doi.org/10.1161/CIRCULATIONAHA.121.056940.
Schroeder I, Scharf C, Zoller M, et al. Charakteristika und Outcome von 70 beatmeten COVID-19-Patienten: Bilanz nach der ersten Welle an einem universitären Zentrum. Anaesthesist. 2021;70(7):573–81. https://doi.org/10.1007/s00101-020-00906-3.
Supady A, Weber E, Rieder M, et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med. 2021;9(7):755–62. https://doi.org/10.1016/S2213-2600(21)00177-6.
Stockmann H, Thelen P, Stroben F, et al. CytoSorb rescue for COVID-19 patients with vasoplegic shock and multiple organ failure: a prospective, open-label, randomized controlled pilot study. Crit Care Med. 2022. https://doi.org/10.1097/CCM.0000000000005493.
Rampino T, Gregorini M, Perotti L, et al. Hemoperfusion with CytoSorb as adjuvant therapy in critically Ill patients with SARS-CoV2 pneumonia. Blood Purif. 2021;50(4–5):566–71. https://doi.org/10.1159/000511725.
Supady A, Zahn T, Kuhl M, et al. Cytokine adsorption in patients with post-cardiac arrest syndrome after extracorporeal cardiopulmonary resuscitation (CYTER)—a single-centre, open-label, randomised, controlled trial. Resuscitation. 2022. https://doi.org/10.1016/j.resuscitation.2022.02.001.
Akin M, Garcheva V, Sieweke JT, et al. Early use of hemoadsorption in patients after out-of hospital cardiac arrest—a matched pair analysis. Ballotta A, ed. PLOS ONE. 2020;15(11):e0241709. https://doi.org/10.1371/journal.pone.0241709
Supady A, Zahn T, Rieder M, et al. Effect of cytokine adsorption on survival and circulatory stabilization in patients receiving extracorporeal cardiopulmonary resuscitation. ASAIO J. 2021. https://doi.org/10.1097/MAT.0000000000001441.
Scharf C, Schroeder I, Paal M, et al. Can the cytokine adsorber CytoSorb® help to mitigate cytokine storm and reduce mortality in critically ill patients? A propensity score matching analysis. Ann Intensive Care. 2021;11(1):115. https://doi.org/10.1186/s13613-021-00905-6.
Rieder M, Duerschmied D, Zahn T, et al. Cytokine adsorption in severe acute respiratory failure requiring veno-venous extracorporeal membrane oxygenation. ASAIO J. 2021;67(3):332–8. https://doi.org/10.1097/MAT.0000000000001302.
Rasch S, Sancak S, Erber J, et al. Influence of extracorporeal cytokine adsorption on hemodynamics in severe acute pancreatitis: results of the matched cohort pancreatitis cytosorbents inflammatory cytokine removal (PACIFIC) study. Artif Organs. 2022. https://doi.org/10.1111/aor.14195.
Wilhelmi M. Pilotstudie zur Outcome-Analyse bei Einsatz einer Cytokin-Hämoadsorption bei schwerstverletzten Polytraumapatienten. Published online 2018. https://cytosorb-therapy.com/wp-content/uploads/2018/12/2018_Wilhelmi_Polytrauma_DIVI_Poster1.pdf
Barzilay E, Kessler D, Berlot G, Gullo A, Geber D, Ben ZI. Use of extracorporeal supportive techniques as additional treatment for septic-induced multiple organ failure patients. Crit Care Med. 1989;17(7):634–7. https://doi.org/10.1097/00003246-198907000-00007.
Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255–73. https://doi.org/10.1056/NEJMra2026131.
Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58(4):867–74. https://doi.org/10.1097/01.ta.0000158244.69179.94.
Rimmelé T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care Lond Engl. 2011;15(1):205. https://doi.org/10.1186/cc9411.
Ricci Z, Romagnoli S, Reis T, Bellomo R, Ronco C. Hemoperfusion in the intensive care unit. Intensive Care Med. 2022;48(10):1397–408. https://doi.org/10.1007/s00134-022-06810-1.
Kielstein JT, Zarbock A. Is this the beginning of the end of cytokine adsorption? Crit Care Med. 2022;50(6):1026–9. https://doi.org/10.1097/CCM.0000000000005509.
Kellum JA, Song M, Venkataraman R. Hemoadsorption removes tumor necrosis factor, interleukin-6, and interleukin-10, reduces nuclear factor-kappaB DNA binding, and improves short-term survival in lethal endotoxemia. Crit Care Med. 2004;32(3):801–5. https://doi.org/10.1097/01.ccm.0000114997.39857.69.
Harm S, Schildböck C, Hartmann J. Cytokine removal in extracorporeal blood purification: an in vitro study. Blood Purif. 2020;49(1–2):33–43. https://doi.org/10.1159/000502680.
Honoré PM, Matson JR. Extracorporeal removal for sepsis: acting at the tissue level–the beginning of a new era for this treatment modality in septic shock. Crit Care Med. 2004;32(3):896–7. https://doi.org/10.1097/01.ccm.0000115262.31804.46.
Harm S, Falkenhagen D, Hartmann J. Pore size–a key property for selective toxin removal in blood purification. Int J Artif Organs. 2014;37(9):668–78. https://doi.org/10.5301/ijao.5000354.
König C, Röhr AC, Frey OR, et al. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int J Artif Organs. 2019;42(2):57–64. https://doi.org/10.1177/0391398818812601.
Funding
Open Access funding enabled and organized by Projekt DEAL. There was no funding for this study.
Author information
Authors and Affiliations
Contributions
SB wrote the first draft of the manuscript, searched the literature, extracted data, and analysed data; HL finalized the manuscript, searched the literature, extracted data, analysed data; CVB revised the manuscript and extracted data, ZT searched the literature and revised the manuscript, AM designed the study, supervised the work and revised the manuscript, BS designed the study and supervised the work, analysed data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable in a meta-analysis.
Competing interests
BMWS received lecture fees and honoraria from ADVITOS, Amgen, Bayer Vital, Berlin Chemie, CytoSorbents, Daichii Sankyo, Miltenyi, Pocard. All other authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Fig. S1:
30-day mortality by different diagnoses. Fig. S2: In-hospital mortality by different diagnoses. Fig. S3: ICU mortality by different diagnoses. Fig. S4: Risk of Bias in Non-randomized Studies of Interventions. Fig. S5: Risk of Bias in randomized trials. Fig. S6: Funnel plot for nPSM. Fig. S7: Funnel plot for PSM. Fig. S8: Funnel plot for RCT. Table S1: Baseline SOFA, SAPS-2, APACHE-2 and EuroScore-2 (difference: treatment group – control group). Table S2: CRP and PCT before and first reported after treatment (difference: treatment group – control group). Table S3: Lactate and IL-6 before and first reported after treatment (difference: treatment group – control group).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Becker, S., Lang, H., Vollmer Barbosa, C. et al. Efficacy of CytoSorb®: a systematic review and meta-analysis. Crit Care 27, 215 (2023). https://doi.org/10.1186/s13054-023-04492-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04492-9