Abstract
Introduction
Immunocompromised (IC) patients mount poor immune responses to vaccination. Higher-dose coronavirus disease 2019 (COVID-19) vaccines may offer increased immunogenicity.
Methods
A pairwise meta-analysis of 98 studies reporting comparisons of mRNA-1273 (50 or 100 mcg/dose) and BNT162b2 (30 mcg/dose) in IC adults was performed. Outcomes were seroconversion, total and neutralizing antibody titers, and cellular immune responses.
Results
mRNA-1273 was associated with a significantly higher seroconversion likelihood [relative risk, 1.11 (95% CI, 1.08, 1.14); P < 0.0001; I2 = 66.8%] and higher total antibody titers [relative increase, 50.45% (95% CI, 34.63%, 66.28%); P < 0.0001; I2 = 89.5%] versus BNT162b2. mRNA-1273 elicited higher but statistically nonsignificant relative increases in neutralizing antibody titers and cellular immune responses versus BNT162b2.
Conclusion
Higher-dose mRNA-1273 had increased immunogenicity versus BNT162b2 in IC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Vaccines against coronavirus disease 2019 (COVID-19) generally elicit poor humoral and cellular immune responses in immunocompromised (IC) patients, which puts this vulnerable population at increased risk of severe COVID-19. |
Because IC patients are often excluded from randomized controlled trials, there are limited data on which of the widely used mRNA COVID-19 vaccines is most immunogenic in this population. |
We performed a systematic literature review and a pairwise meta-analysis of studies reporting comparisons of mRNA-1273 (primary series, 100 mcg mRNA/dose; booster, 50 mcg/dose) and BNT162b2 (primary series and booster, 30 mcg mRNA/dose) in IC patients to evaluate differences in immunogenicity between these 2 mRNA COVID-19 vaccines. |
Vaccination with mRNA-1273 was associated with a significantly higher likelihood of seroconversion (1.11 times) and significantly higher total antibody titers (50% relative increase) in IC patients than vaccination with BNT162b2. |
Our findings provide data that may inform COVID-19 vaccination strategy for high-risk, immunosuppressed populations. |
Introduction
Several vaccines against SARS-CoV-2, which causes COVID-19, were developed in response to the COVID-19 pandemic [1]. Two vaccines using novel messenger ribonucleic acid (mRNA) technology were approved for use against COVID-19 [1]: mRNA-1273 (Spikevax®, Moderna, Inc., Cambridge, MA, USA) [2] and BNT162b2 (Comirnaty®, Pfizer/BioNTech, New York, NY, USA/Mainz, Germany) [3]. Both mRNA-1273 and BNT162b2 administered in a 2-dose series significantly reduced symptomatic infections and hospitalizations in immunocompetent populations evaluated in pivotal studies [4, 5]. The mRNA-1273 and BNT162b2 2-dose primary series were also shown to elicit high neutralizing antibody titers against the spike protein of SARS-CoV-2, as well as high rates of seroconversion in the general population [6, 7].
People who are immunocompromised (IC) generally mount poor immune responses to vaccination because of their immunocompromising conditions or therapies used to treat their underlying diseases, rendering them susceptible to infections [8]. IC populations include but are not limited to patients with cancer, autoimmune diseases, HIV, or primary or secondary immune system deficiencies; solid organ transplant recipients; and patients receiving immunosuppressive therapies (e.g., B-cell-depleting agents such as anti-CD20 monoclonal antibodies) [9].
Approximately 10 million people in the United States are considered to be IC [10]; however, people with IC conditions were excluded from participating in phase 2/3 clinical trials of mRNA-1273 and BNT162b2 [4, 5]. Observational studies have demonstrated that IC populations are at increased risk of COVID-19–related morbidity and mortality compared with the general population [8, 11,12,13]. One study showed that nearly half (44%) of COVID-19–associated hospitalizations among vaccinated people (i.e., breakthrough hospitalizations) occurred in IC individuals [14]. IC patients are also at higher risk of longer courses of infection [15,16,17,18,19,20,21,22] and viral evolution [15,16,17,18, 20, 23, 24]. Poor humoral immune responses also exacerbate risks posed by new SARS-CoV-2 variants [25,26,27,28,29,30,31].
High-dose influenza vaccines have been shown to elicit greater immune responses compared with standard-dose vaccines in IC populations [32,33,34,35,36,37]. Higher-dose mRNA COVID-19 vaccines may offer similar benefits. In addition to differences in the lipid nanoparticle component of the vaccines, the primary series of mRNA-1273 contains over 3 times the amount of mRNA compared with BNT162b2 (100 mcg/dose vs. 30 mcg/dose) and the booster nearly 2 times (50 mcg/dose vs. 30 mcg/dose) [2, 3, 38, 39]. Humoral immune responses to COVID-19 vaccines have been shown to correlate with clinical efficacy or effectiveness in general [40,41,42] and IC populations [43, 44]. Although randomized controlled trials (RCTs) evaluating the comparative immunogenicity of mRNA COVID-19 vaccines in IC populations are lacking, observational studies suggest that there are differences in the immune responses elicited by the mRNA COVID-19 vaccines in IC populations [28, 45], which may affect protection from severe COVID-19 in these vulnerable populations.
National immunization technical advisory groups, including the Advisory Committee on Immunization Practices (ACIP) in the United States, make recommendations on the best use of available vaccines in specific populations [46]. ACIP evaluates available evidence according to the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework to formulate vaccine recommendations [47, 48]. For example, a systematic literature review (SLR) and pairwise meta-analysis was performed by ACIP to evaluate the evidence for high-dose influenza vaccines compared with standard-dose influenza vaccines in adults ≥ 65 years old [49]. The results were evaluated using the GRADE framework and ultimately supported a recommendation for high-dose influenza vaccines in adults ≥ 65 years old [49, 50].
Determining which of the mRNA-based COVID-19 vaccines offer the highest protection from disease is essential in preventing poor COVID-19–related outcomes in highly susceptible IC populations. Data on the comparative immunogenicity of mRNA-1273 and BNT162b2 in IC populations are therefore urgently needed to inform public health policy. In this SLR and pairwise meta-analysis, we have compared seroconversion rates, total and neutralizing anti-spike antibody titers, and cellular immunity levels in IC individuals after vaccination with 2 or 3 doses of mRNA-1273 or BNT162b2. We also applied the GRADE framework to address the following healthcare question: does the 2-dose mRNA-1273 COVID-19 vaccine primary series (100 mcg mRNA/dose) or primary series and booster (50 or 100 mcg mRNA/dose) have greater immunogenicity in IC populations compared with the 2-dose BNT162b2 COVID-19 vaccine primary series or primary series and booster (30 mcg mRNA/dose irrespective of dose type)?
Methods
The SLR was performed per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 framework [51]. A separate meta-analysis evaluating the comparative clinical effectiveness of mRNA-1273 and BNT162b2 has been performed using data extracted from the same SLR and published separately [52]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Search Strategy
As previously described [52], the main search was conducted in the World Health Organization COVID-19 Research Database on April 14, 2022, and updated on December 19, 2022. Table S1 lists the search strings.
Study Selection Criteria
Titles and abstracts were screened against inclusion criteria by two independent reviewers in level 1. Full texts were evaluated against selection criteria in level 2. A third reviewer arbitrated conflicts between reviewers.
A summary of the population, intervention/exposure, comparison, and outcomes described below is shown in Table S2. Studies were included if they were clinical trials, observational studies, or any real-world evidence published as manuscripts, letters, commentaries, abstracts, or posters reporting immunogenicity outcomes in IC individuals ≥ 18 years of age vaccinated with a homologous primary series of mRNA-1273 or BNT162b2 or the primary series followed by a homologous booster within the same study. We defined the primary series as either 2 doses of mRNA-1273 (100 mcg mRNA/dose) or BNT162b2 (30 mcg mRNA/dose) or used the definition of primary series reported on a per-study basis due to globally varying recommendations for a third vaccine dose in IC populations over time. The booster dose was defined as a homologous third dose of mRNA-1273 (50 or 100 mcg mRNA/dose) or BNT162b2 (30 mcg mRNA/dose). People belonging to clinically extremely vulnerable (CEV) groups 1 or 2 [9], which included transplant recipients, patients with cancer, primary immunodeficiencies, dialysis or severe kidney disease, poorly controlled HIV infection, or autoimmune diseases requiring immunosuppressive therapy, were considered to be IC. Recently published SLRs on the same topic were cross-checked to ensure relevant articles were not omitted. Studies reporting outcomes in pregnant women, current or former smokers, or physically inactive people and those with a heterologous vaccination schedule (i.e., mix of mRNA-1273 and BNT162b2), only safety data, or study protocols or economic models were excluded.
Outcomes
Immunogenicity outcomes were selected based on immune correlates of protection against SARS-CoV-2 investigated in preclinical and clinical studies of COVID-19 vaccines [42, 53, 54]. These correlates of protection are associated with lower risk of severe infection at the population level; however, the threshold of protection for total antibody and neutralizing antibodies has yet to be established [40, 54]. Regulatory authorities have authorized mRNA COVID-19 vaccines for emergency use among different age groups, additional primary series doses for IC populations, and booster doses through immunobridging (i.e., predicting vaccine effectiveness based on immunogenicity demonstrated in new populations); the correlates of immunity used were seroresponse rates and neutralizing antibody titers [55,56,57].
The primary outcome was the percentage of patients achieving seroconversion after vaccination with 2 or 3 doses of mRNA-1273 compared with BNT162b2. Seroconversion was defined as the presence of SARS-CoV-2 anti-spike antibodies above the cutoff value indicated by the specifications of the manufacturer of the assay used to measure antibody titers following vaccination, or as defined in each study based on an evaluation of correlation with plaque reduction neutralization tests. Details on assays used in each study are provided in Table S3. Secondary outcomes were total anti-spike binding antibody or immunoglobulin G (IgG) titers (Table S4), neutralizing anti-spike antibody titers (Table S5), and cellular immune response based on interferon (IFN)-γ or interleukin (IL) levels or CD4+/CD8+ T-cell levels (Table S6).
Outcomes reported after the homologous 2-dose primary series were included in the analysis. If not reported, outcomes following the homologous third dose (booster or third full dose) were included. For studies reporting multiple time points, outcomes assessed 2 weeks after the second dose of the primary series (if only 2 doses were given) or the third dose administered were preferentially included. If outcomes 2 weeks after the last dose administered were not reported, outcomes assessed at time points ≥ 2 weeks after the final dose were considered instead. For studies reporting multiple IC populations, data were included from populations with the highest number of patients, events, or measured antibody titers for each study. Total patient population and event numbers or rates were required to be reported for seroconversion, and the mean or median was required to be reported for total and neutralizing anti-spike antibody titers. If the total population size per vaccine arm was not reported, the study was excluded from the meta-analysis. Whenever available, antibody titers assessed by the Roche Elecsys platform and in COVID-19–naïve populations were included in the meta-analysis.
Total binding antibody titers were preferentially used for the total anti-spike antibody outcome if reported; otherwise, anti-spike IgG titers were used. Data for the cellular immune response outcome were preferentially taken from IFN-γ-producing or IL-producing T-cell assays or concentrations, followed by CD4+/CD8+ T-cell counts or mean spot-forming units from ELISpot assays. If available, composite scores of the data described above were considered first, followed by IFN-γ levels and other IL levels. Studies were required to report mean or median levels. Distribution statistics were imputed from available data if they were not reported [58].
Data Extraction and Quality Assessment
Publication details, study and patient characteristics, vaccine type and vaccination status, IC condition, background anti-CD20 monoclonal antibody treatment, and immunogenicity data were extracted. Risk of bias (RoB) was assessed in accordance with Cochrane review guidelines [59] using version 2 of the RoB 2 tool [60] for randomized studies and the Newcastle–Ottawa scale [61] for observational studies. Observational studies with < 7 versus ≥ 7 stars were considered to have serious versus no serious RoB, respectively. Evidence was evaluated based on the GRADE framework [47, 48].
Statistical Analysis
Irrespective of assay type, random-effects meta-analysis models were used to pool risk ratios [RRs) across studies, and absolute effects as risk difference per 100,000 individuals were calculated across studies for the seroconversion outcome. Inverse variance weights were calculated for individual studies with the DerSimonian–Laird method [62]. Relative increase and corresponding standard errors in total anti-spike binding or IgG antibody titers, neutralizing antibody titers, and cellular immune response outcomes were calculated to compare mRNA-1273 versus BNT162b2 following the Dubey method, as suggested in the Cochrane Handbook [58, 63]. Chi-square testing to evaluate heterogeneity across studies was performed [64]. The I2 statistic was estimated (0–100%) and interpreted as follows: 0–40%, no evidence of heterogeneity or heterogeneity might not be important; > 40–60%, evidence of moderate heterogeneity; > 60–75%, evidence of substantial heterogeneity; and > 75%, evidence of considerable heterogeneity.
Outcomes were analyzed separately for RCTs and nonrandomized studies. To reduce heterogeneity introduced by differences in the underlying IC condition and therapies used to treat the IC condition, 2 subgroup analyses were conducted. The first subgroup analysis analyzed, separately, patients with autoimmune disease, solid organ transplant recipients, patients with solid tumors, and patients with hematologic malignancies. In the second subgroup analysis, outcomes were further analyzed separately by prior anti-CD20 treatment versus no treatment in IC patients overall and among patients with autoimmune disease and hematologic malignancies.
Results
Overview of Included Studies
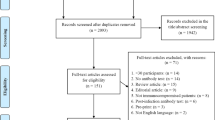
Of the 5745 nonduplicate articles identified in the main searches, 130 articles reporting immunogenicity outcomes in IC patients vaccinated with mRNA-1273 versus BNT162b2 in the same study were included in the SLR. Of these, 98 articles were included in the pairwise meta-analysis (Fig. 1). Reasons for excluding articles from the meta-analysis were that the data were not suitable for meta-analysis (e.g., only event rates reported or data not reported by vaccine arm separately; n = 13), patients received either a single dose of vaccine or a heterologous vaccine series (n = 9), the population did not meet CEV groups 1 and 2 or age inclusion criteria (n = 7), and duplicate studies (e.g., preprint version of another included study; n = 3).
PRISMA flow diagram. Searches were first performed on April 14, 2022, followed by an update on December 19, 2022. *Databases searched include ICTRP, Embase, EuropePMC, medRxiv, Web of Science, ProQuest Central, Academic Search Complete, Scopus, and COVIDWHO. **Includes internal documents from Moderna and recently published SLRs. ICTRP International Clinical Trials Registry Platform, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SLR systematic literature review, WHO World Health Organization
Characteristics of the studies included in the meta-analysis by outcome are provided in Tables S3−S6. Of the 98 articles included in the pairwise meta-analysis overall, 1 study was an RCT [65] and the remaining 97 were nonrandomized studies. A total of 79 nonrandomized studies comprising 23,135 patients vaccinated with mRNA-1273 (n = 9664) or BNT162b2 (n = 13,471) were analyzed for the overall seroconversion outcome; Forty-five nonrandomized studies comprising 8913 patients vaccinated with mRNA-1273 (n = 3038) or BNT162b2 (n = 5875) and 1 RCT comprising 50 patients vaccinated with mRNA-1273 (n = 24) or BNT162b2 (n = 26) were analyzed for the overall total anti-spike binding antibody or IgG titer outcome; 7 nonrandomized studies, comprising 592 patients vaccinated with mRNA-1273 (n = 236) or BNT162b2 (n = 356), were analyzed for the overall neutralizing anti-spike antibody titer outcome; and 14 nonrandomized studies comprising 2583 patients vaccinated with mRNA-1273 (n = 878) or BNT162b2 (n = 1705), were analyzed for the overall cellular immune response outcome. Most studies evaluated the 2-dose primary series (n = 81, 82.7%). Patients received 2 doses of the primary series followed by 1 booster dose in 17 studies (17.3%) [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. Most studies did not specify whether the mRNA-1273 booster dose was a true booster (i.e., 50 mcg mRNA/dose) or a third dose of the primary series (i.e., 100 mcg mRNA/dose).
Assessment of RoB was performed, and nearly half of the studies (n = 47, 48.0%) were determined to have no serious RoB (Table S7; Table S8). Forty-five studies (45.9%) had serious RoB, largely because non-IC cohorts were not included or not described if included, only 1 cohort was included, or cohort comparability was not assessed or described if multiple cohorts were included. RoB was not assessed for 6 articles (6.1%) because they were abstracts or research letters.
Seroconversion
The meta-analysis of 79 nonrandomized studies overall found that seroconversion was significantly more likely with mRNA-1273 compared with BNT162b2 in IC patients [RR, 1.11 (95% CI, 1.08, 1.14); P < 0.0001; Table 1]. The heterogeneity between studies was considered substantial (I2 = 66.8%). Expressed as absolute differences, we found that mRNA-1273 vaccination would result in seroconversion of 8170.28 more IC patients per 100,000 IC patients (95% CI, 6279.09, 10,061.47; P < 0.0001; I2 = 60.3%) compared with BNT162b2 vaccination.
Similar trends in seroconversion were observed in IC patients analyzed by disease subgroup (Table 2; Fig. 2). Among solid organ transplant recipients, mRNA-1273 was significantly more likely to result in seroconversion compared with BNT162b2 [RR, 1.29 (95% CI, 1.19, 1.39); P < 0.0001; I2 = 43.5%]. In patients with autoimmune disease, seroconversion was significantly more likely for mRNA-1273 compared with BNT162b2 [RR, 1.07 (95% CI, 1.03, 1.11); P = 0.0002; I2 = 58.1%]. Patients with hematologic malignancies were also significantly more likely to seroconvert following mRNA-1273 vaccination versus BNT162b2 [RR, 1.16 (95% CI, 1.09, 1.24); P < 0.0001; I2 = 57.0%]. mRNA-1273 was not associated with a statistically significant difference in likelihood of seroconversion compared with BNT162b2 in patients with solid tumors.
GRADE analysis found that the certainty of evidence of the seroconversion outcome overall and in the solid organ transplant and autoimmunity subgroups was downgraded to type 4 (very low) due to imprecision and indirectness because different assays and antibody thresholds were used to assess and define seroconversion across the studies analyzed. Evidence certainty in the subgroup of patients with hematologic malignancies was retained as type 3 (i.e., the maximum certainty possible for nonrandomized studies), because there was a strong association in seroconversion likelihood and no issues with imprecision.
Total Anti-spike Binding Antibody or IgG Titers
mRNA-1273 elicited a statistically significant relative increase in total anti-spike binding antibody or IgG titers versus BNT162b2 [50.45% (95% CI, 34.63%, 66.28%); P < 0.0001] in 45 nonrandomized studies of IC patients overall (Table 1). However, heterogeneity was considerable between studies (I2 = 89.5%).
Subgroup analyses showed that patients with solid organ transplant, solid tumors, and autoimmune disease vaccinated with mRNA-1273 compared with BNT162b2 had 335.04%, 104.82%, and 74.75% higher relative increases in total anti-spike binding antibody or IgG titers, respectively (Table 3). These findings were statistically significant (all P < 0.05). Moderate and considerable heterogeneity was found in the solid organ transplant (I2 = 64.7%) and autoimmune disease (I2 = 91.8%) subgroups, with no evidence of heterogeneity in patients with solid tumors (I2 = 39.6%). Compared with BNT162b2, a higher relative increase in total anti-spike binding antibody or IgG titers in patients with hematologic malignancies was observed in the mRNA-1273 group; however this finding was not statistically significant (Table 3).
GRADE evidence certainty for the total binding antibody or IgG titer outcome in IC patients overall and by autoimmune disease, solid organ transplant, and solid tumor subgroups was type 3 (low); lower grading because of imprecision was offset by higher grading due to a strong association in the relative increase in total anti-spike binding antibody or IgG titers.
Neutralizing Anti-spike Titers
The SARS-CoV-2 variant against which the neutralization assays were performed was not reported in 5 studies [80, 82,83,84,85]; neutralization was evaluated against delta, omicron, and early WA1/2020 variants in 1 study [86] and against delta, omicron, and D614G/ancestral variants in another study [87]. When assessed in IC patients overall from 7 nonrandomized studies, neutralizing anti-spike titers were not statistically significantly different in patients who received mRNA-1273 compared with BNT162b2 (Table 1). mRNA-1273 was associated with statistically significant relative increases in neutralizing anti-spike titers compared with BNT162b2 [436.78% (95% CI, 164.47%, 709.08%); P = 0.0017] in the subgroup of solid organ transplant recipients (Table 4). No evidence of heterogeneity was observed (I2 = 0%). No statistically significant differences in neutralizing anti-spike titers between vaccines were observed for the remaining subgroups (Table 4).
GRADE analysis found the certainty of evidence for the neutralizing anti-spike titer outcome in IC patients overall to be type 4 (very low) because of imprecision among the nonrandomized studies.
Cellular Immune Response
The meta-analysis of 14 nonrandomized studies reporting cellular immune responses in IC patients overall found no statistically significant differences in the relative increase or decrease in the cellular immune response elicited by mRNA-1273 versus BNT162b2 (Table 1). Consistent with the meta-analysis of cellular immune response in IC patients overall, no statistically significant findings were observed in disease subgroups (Table 5).
Effect of Anti-CD20 Treatment on Outcomes
B-cell–depleting therapy through anti-CD20–specific monoclonal antibodies used to treat some patients with autoimmune disease or hematologic malignancies can severely impair the development of immune responses [88]. Therefore, and to account for heterogeneity between studies, we performed a subgroup analysis of patients with and without anti-CD20 monoclonal antibody treatment overall and in patients with autoimmune disease and hematologic malignancies. Vaccination with mRNA-1273 was associated with higher rates of seroconversion (Table S9) and elicited higher relative increases in total anti-spike binding antibody or IgG titers (Table S10), neutralizing anti-spike antibody titers (Table S11), and cellular immune responses (Table S12) in IC patients who received anti-CD20 treatment compared with BNT162b2. Generally similar trends were observed in patients with autoimmune disease and hematologic malignancies.
Discussion
In this SLR and pairwise meta-analysis of IC patients ≥ 18 years of age vaccinated with mRNA COVID-19 vaccines, IC patients who received mRNA-1273 were significantly more likely to achieve seroconversion than those who received BNT162b2. Consistent with the seroconversion outcome, mRNA-1273 was also associated with a statistically significant relative increase in total anti-spike binding antibody or IgG titers over BNT162b2. Neutralizing anti-spike antibody titers were approximately 35% higher in IC patients vaccinated with mRNA-1273 versus BNT162b2; however, this result was not statistically significant. We also assessed cellular immune responses, which have been associated with protection against initial SARS-CoV-2 infection and viral clearance and may be critical for long-lasting immunity to SARS-CoV-2 [89]. Although no statistically significant differences in cellular immunity were observed between mRNA-1273 and BNT162b2 in this meta-analysis, the cellular immune response was approximately 15% higher in IC patients overall who were vaccinated with mRNA-1273 versus BNT162b2. Because only 14 nonrandomized studies, each with differing metrics of cellular immunity, were included in the meta-analysis for this outcome, it is possible that analyzing a larger number of studies with similar outcome definitions may have yielded clearer results.
Consistent with the practices of ACIP, the GRADE framework was used to evaluate evidence in this meta-analysis. Evidence for mRNA-1273 versus BNT162b2 vaccination was rated to be type 4 (very low) for the seroconversion, neutralizing antibody titer, and cellular immune response outcomes and type 3 (low) for the total antibody titer outcome (Table 6). Because IC populations are often excluded from RCTs, all but 1 of the 98 studies included in our meta-analysis were observational, nonrandomized studies, so the maximum evidence certainty achievable was type 3 [GRADE sets the maximum certainty for evidence derived from nonrandomized studies to type 3 (low) [47]]. Logistical challenges, such as the rapid development and administration of mRNA COVID-19 vaccines in both general and IC populations, meant that RCTs designed specifically to test comparative immunogenicity of mRNA COVID-19 vaccines in IC patients were not feasible to conduct. Despite obligatory reliance on nonrandomized studies, we were able to consistently show strong associations between mRNA-1273 and the likelihood of achieving seroconversion and relative increases in total antibody titers in the observational studies identified in the SLR.
Anti-CD20 monoclonal antibody therapy is used to treat some autoimmune diseases (e.g., multiple sclerosis) [90] and hematologic malignancies characterized by aberrant B-cell proliferation (e.g., non-Hodgkin lymphoma) [91]. Anti-CD20 therapy depletes B cells, possibly rendering patients receiving this therapy susceptible to SARS-CoV-2 infection and severe COVID-19 because of impaired humoral immune responses [88]. Our meta-analysis included studies of patients both with and without anti-CD20 treatment, which, while increasing the heterogeneity observed in some outcomes, allowed the impact of anti-CD20 treatment on differential immunogenicity of mRNA-1273 and BNT162b2 to be analyzed. Consistent with the overall IC population, mRNA-1273 was more likely to result in seroconversion and induce higher humoral and cellular immune responses than BNT162b2 in patients with anti-CD20 treatment. Although our findings were not statistically significant, in the absence of robust RCTs evaluating mRNA COVID-19 vaccines in IC populations receiving anti-CD20 therapy, they suggest that mRNA-1273 may be more immunogenic than BNT162b2 in this population at even greater risk for severe COVID-19 compared with the overall IC population.
Limitations of our study are that non-English studies were excluded from the SLR and that publication bias was not assessed in the meta-analysis. Furthermore, we observed considerable heterogeneity, in part due to differing study designs (e.g., case control, cohort), differences in the number of vaccine doses or booster doses administered by study, as well as lack of detail regarding mRNA dosage in reported mRNA-1273 booster doses, differing outcome definitions between studies, and differences in methods and assays used to measure antibody titers. Changing prevalence of variants of concern over time and differing immune responses to the variants as well as the heterogenous nature of IC conditions and background treatments (e.g., anti-CD20 therapy, immunoglobulin replacement therapy) also contributed to the heterogeneity we observed. To address some of these limitations, we conducted subgroup analyses by IC condition and anti-CD20 treatment subgroups as previously discussed, preferentially included antibody titers from the Roche Elecsys platform where available, and used relative increase or decrease in humoral and cellular immunity outcomes to mitigate the heterogeneity caused by multiple outcome definitions and cutoffs. Although these strategies reduced heterogeneity, more data are needed to address all the limitations of our meta-analysis.
As previously described [52], differences in prescribing behavior or the impact of vaccine choice could not be accounted for in our meta-analysis. Besides mRNA dose in mRNA-1273 and BNT162b2, other differences in vaccine formulation or delivery, such as the type of lipid nanoparticle encapsulating the mRNA, mRNA translation efficiency, and vaccination schedule (4 vs. 3 weeks between doses of the primary series), may have impacted immunogenicity.
Conclusions
Our meta-analysis of mostly observational studies showed that vaccination with a higher-dose mRNA-1273 primary series (100 mcg mRNA/dose) or primary series and booster (50 or 100 mcg mRNA/dose) was more likely to result in seroconversion and elicited higher total anti-spike antibody titers than the BNT162b2 primary series or primary series and booster (30 mcg mRNA/dose irrespective of dose type). These findings suggest that immune responses to SARS-CoV-2 vaccination could be optimized in IC patients by selecting an appropriate mRNA vaccine and can help inform vaccine strategy in high-risk, immunosuppressed populations.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20:817–38.
Spikevax (mRNA-1273). Full Prescribing Information, Moderna, Inc., Cambridge, MA, 2022.
Comirnaty (BNT162b2). Full Prescribing Information, Pfizer/BioNTech, New York, NY, 2022.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–15.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16.
Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–31.
Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–50.
DeWolf S, Laracy JC, Perales MA, et al. SARS-CoV-2 in immunocompromised individuals. Immunity. 2022;55:1779–98.
BC COVID Therapeutics Committee. Practice Tool #2 - Definitions of CEV/Immunosuppressed. BC Centre for Disease Control. Available at: http://www.bccdc.ca/Health-Professionals-Site/Documents/COVID-treatment/PracticeTool2_CEVCriteria.pdf. Accessed 16 Feb 2023.
Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316:2547–8.
Dandachi D, Geiger G, Montgomery MW, et al. Characteristics, comorbidities, and outcomes in a multicenter registry of patients with human immunodeficiency virus and coronavirus disease 2019. Clin Infect Dis. 2021;73:e1964–72.
National Center for Immunization and Respiratory Diseases (NCIRD) Division of Viral Diseases. People with Certain Medical Conditions. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed 16 Feb 2023.
Singson JRC, Kirley PD, Pham H, et al. Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19 - COVID-NET, 10 states, March 2020-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:878–84.
Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022;74:1515–24.
Truong TT, Ryutov A, Pandey U, et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: a consecutive case series. EBioMedicine. 2021;67: 103355.
Hensley MK, Bain WG, Jacobs J, et al. Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: a case study. Clin Infect Dis. 2021;73:e815–21.
Baang JH, Smith C, Mirabelli C, et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis. 2021;223:23–7.
Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–3.
Helleberg M, Niemann CU, Moestrup KS, et al. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis. 2020;222:1103–7.
Khatamzas E, Rehn A, Muenchhoff M, et al. Emergence of multiple SARS-CoV-2 mutations in an immunocompromised host. medRxiv. Preprint posted online April 7, 2023. https://doi.org/10.1101/2021.01.10.20248871.
Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901-12.e9.
Nakajima Y, Ogai A, Furukawa K, et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother. 2021;27:387–9.
Clark SA, Clark LE, Pan J, et al. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell. 2021;184:2605-17.e18.
Kemp SA, Collier DA, Datir RP, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592:277–82.
Kwon JH, Tenforde MW, Gaglani M, et al. mRNA vaccine effectiveness against coronavirus disease 2019 hospitalization among solid organ transplant recipients. J Infect Dis. 2022;226:797–807.
Parker EPK, Desai S, Marti M, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10:e326–8.
Patyna S, Eckes T, Koch BF, et al. Impact of Moderna mRNA-1273 booster vaccine on fully vaccinated high-risk chronic dialysis patients after loss of humoral response. Vaccines (Basel). 2022;10:585.
Quiroga B, Soler MJ, Ortiz A, et al. Anti-spike antibodies 3 months after SARS-CoV-2 mRNA vaccine booster dose in patients on hemodialysis: the prospective SENCOVAC study. Clin Kidney J. 2022;15:1856–64.
Risk M, Hayek SS, Schiopu E, et al. COVID-19 vaccine effectiveness against omicron (B.1.1.529) variant infection and hospitalisation in patients taking immunosuppressive medications: a retrospective cohort study. Lancet Rheumatol. 2022;4:e775–84.
Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022;4:e338–50.
Yang LM, Costales C, Ramanathan M, et al. Cellular and humoral immune response to SARS-CoV-2 vaccination and booster dose in immunosuppressed patients: an observational cohort study. J Clin Virol. 2022;153: 105217.
Caldera F, Hillman L, Saha S, et al. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti-TNF monotherapy: a randomized clinical trial. Inflamm Bowel Dis. 2020;26:593–602.
Colmegna I, Useche ML, Rodriguez K, et al. Immunogenicity and safety of high-dose versus standard-dose inactivated influenza vaccine in rheumatoid arthritis patients: a randomised, double-blind, active-comparator trial. Lancet Rheumatol. 2020;2:e14–23.
Hakim H, Allison KJ, Van de Velde LA, et al. Immunogenicity and safety of high-dose trivalent inactivated influenza vaccine compared to standard-dose vaccine in children and young adults with cancer or HIV infection. Vaccine. 2016;34:3141–8.
Halasa NB, Savani BN, Asokan I, et al. Randomized double-blind study of the safety and immunogenicity of standard-dose trivalent inactivated influenza vaccine versus high-dose trivalent inactivated influenza vaccine in adult hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant. 2016;22:528–35.
McKittrick N, Frank I, Jacobson JM, et al. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med. 2013;158:19–26.
Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs. standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66:1698–704.
Pfizer/BioNTech. Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) Emergency Use Authorization (EUA): Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) and Booster Dose for 12 Years of Age and Older. Pfizer/BioNTech. Available at: https://www.fda.gov/media/167211/download. Accessed 7 Mar 2023.
Moderna Inc. Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) Emergency Use Authorization (EUA): Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) and Booster Dose for 6 Years of Age and Older. Moderna, Inc. Available at: https://assets.modernatx.com/m/4439414c67267464/original/EUA-Fact-Sheet-PI-Providers-Bivalent-Booster-6y.pdf. Accessed 7 Mar 2023.
Khoury DS, Schlub TE, Cromer D, et al. Correlates of protection, thresholds of protection, and immunobridging among persons with SARS-CoV-2 infection. Emerg Infect Dis. 2023;29:381–8.
Regev-Yochay G, Lustig Y, Joseph G, et al. Correlates of protection against COVID-19 infection and intensity of symptomatic disease in vaccinated individuals exposed to SARS-CoV-2 in households in Israel (ICoFS): a prospective cohort study. Lancet Microbe. 2023;4:e309–18.
Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50.
Wijaya R, Johnson M, Campbell N, et al. Predicting COVID-19 infection risk in people who are immunocompromised by antibody testing. Lancet. 2023;402:99–102.
Ahmed S, Mehta P, Paul A, et al. Postvaccination antibody titres predict protection against COVID-19 in patients with autoimmune diseases: survival analysis in a prospective cohort. Ann Rheum Dis. 2022;81:868–74.
Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9: 100178.
National Center for Immunization and Respiratory Diseases. ACIP Recommendations. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/vaccines/acip/recommendations.html. Accessed 24 Apr 2023.
Schünemann H, Brożek J, Guyatt G, Oxman A, eds. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach: The GRADE Working Group; 2013.
Ahmed F, Temte JL, Campos-Outcalt D, Schünemann HJ. Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171–6.
National Center for Immunization and Respiratory Diseases. GRADE: Higher Dose and Adjuvanted Influenza Vaccines for Persons Aged ≥65 Years. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/vaccines/acip/recs/grade/influenza-older-adults.html. Accessed 14 June 2023.
Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices - United States, 2022–23 influenza season. MMWR Recomm Rep. 2022;71:1–28.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Wang X, Haeussler K, Spellman A, et al. Comparative effectiveness of mRNA-1273 and BNT162b2 COVID-19 vaccines in immunocompromised individuals: a systematic review and meta-analysis using the GRADE framework. medRxiv. Preprint posted online April 6, 2023. https://doi.org/10.1101/2023.04.05.23288195.
Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11.
National Center for Immunization and Respiratory Diseases. Science Brief: SARS-CoV-2 Infection-Induced and Vaccine-Induced Immunity. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html#print. Accessed 20 July 2023.
US Food and Drug Administration. Emergency Use Authorization for Moderna COVID-19 Vaccine Review Memo 04182023. Available at: https://www.fda.gov/media/167306/download. Accessed 20 July 2023.
US Food and Drug Administration. Emergency Use Authorization for Pfizer-BioNTech COVID-19 Vaccine Review Memo 04282023. Available at: https://www.fda.gov/media/167669/download. Accessed 20 July 2023.
Gilbert PB, Donis RO, Koup RA, et al. A COVID-19 milestone attained - a correlate of protection for vaccines. N Engl J Med. 2022;387:2203–6.
Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022): Cochrane; 2022.
Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022): Cochrane; 2022.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 16 Feb 2023.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Dubey SD, Lehnhoff RW, Radike AW. A statistical confidence interval for true per cent reduction in caries-incidence studies. J Dent Res. 1965;44:921–3.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Speich B, Chammartin F, Abela IA, et al. Antibody response in immunocompromised patients after the administration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine BNT162b2 or mRNA-1273: a randomized controlled trial. Clin Infect Dis. 2022;75:e585–93.
Abid MB, Rubin M, Ledeboer N, et al. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell. 2022;40:340–2.
Bagacean C, Letestu R, Al-Nawakil C, et al. Humoral response to mRNA anti-COVID-19 vaccines BNT162b2 and mRNA-1273 in patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:207–11.
Barmettler S, DiGiacomo DV, Yang NJ, et al. Response to severe acute respiratory syndrome coronavirus 2 initial series and additional dose vaccine in patients with predominant antibody deficiency. J Allergy Clin Immunol Pract. 2022;10:1622-34.e4.
Chaekal OK, Gomez-Arteaga A, Chen Z, et al. Predictors of COVID-19 vaccination response after in-vivo T-cell-depleted stem cell transplantation. Transplant Cell Ther. 2022;28:618.e1-e10.
Chiang TP, Alejo JL, Mitchell J, et al. Heterologous Ad.26.COV2.S versus homologous BNT162b2/mRNA-1273 as a third dose in solid organ transplant recipients seronegative after two-dose mRNA vaccination. Am J Transplant. 2022;22:2254–60.
Chung A, Banbury B, Vignali M, et al. Antibody and T-cell responses by ultra-deep T-cell receptor immunosequencing after COVID-19 vaccination in patients with plasma cell dyscrasias. Br J Haematol. 2022;199:520–8.
Denault E, Nakajima E, Naranbhai V, et al. Immunogenicity of SARS-CoV-2 vaccines in patients with breast cancer. Ther Adv Med Oncol. 2022;14:17588359221119370.
Maillard A, Redjoul R, Klemencie M, et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood. 2022;139:134–7.
Strauss AT, Chang A, Alejo JL, et al. Severe acute respiratory syndrome coronavirus 2 antibody response to a third dose of homologous messenger RNA vaccination in liver transplantation recipients. Liver Transpl. 2022;28:1393–6.
Stumpf J, Schwöbel J, Karger C, et al. Anti-SARS-CoV-2 revaccination success in kidney transplant recipients with no initial humoral response is linked to primary vaccine type. Front Med. 2022;9:910987.
Thompson MA, Hallmeyer S, Fitzpatrick VE, et al. Real-world third COVID-19 vaccine dosing and antibody response in patients with hematologic malignancies. J Patient Cent Res Rev. 2022;9:149–57.
Watanabe M, Yakushijin K, Funakoshi Y, et al. A third dose COVID-19 vaccination in allogeneic hematopoietic stem cell transplantation patients. Vaccines (Basel). 2022;10:1830.
Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–2.
Gallais F, Renaud-Picard B, Solis M, et al. Torque teno virus DNA load as a predictive marker of antibody response to a three-dose regimen of COVID-19 mRNA-based vaccine in lung transplant recipients. J Heart Lung Transplant. 2022;41:1429–39.
Loubet P, Wittkop L, Ninove L, et al. One-month humoral response following two or three doses of messenger RNA coronavirus disease 2019 vaccines as primary vaccination in specific populations in France: first results from the Agence Nationale Recherche contre le Sida (ANRS)0001S COV-POPART cohort. Clin Microbiol Infect. 2022;29:388.e1-e8.
Quiroga B, Soler MJ, Ortiz A, et al. Humoral response to third dose of SARS-CoV-2 vaccines in the CKD spectrum. Clin J Am Soc Nephrol. 2022;17:872–6.
Vergori A, Cozzi Lepri A, Cicalini S, et al. Immunogenicity to COVID-19 mRNA vaccine third dose in people living with HIV. Nat Commun. 2022;13:4922.
Andreica I, Blazquez-Navarro A, Sokolar J, et al. Different humoral but similar cellular responses of patients with autoimmune inflammatory rheumatic diseases under disease-modifying antirheumatic drugs after COVID-19 vaccination. RMD Open. 2022;8: e002293.
Garcia-Cirera S, Calvet J, Berenguer-Llergo A, et al. Glucocorticoids’ treatment impairs the medium-term immunogenic response to SARS-CoV-2 mRNA vaccines in systemic lupus erythematosus patients. Sci Rep. 2022;12:14772.
Manjappa S, Phi HQ, Lee LW, et al. Humoral and cellular immune response to COVID-19 vaccination in patients with chronic graft-versus-host disease on immunosuppression. Transplant Cell Ther. 2022;28:784.e1-.e.9.
Chang A, Akhtar A, Linderman SL, et al. Humoral responses against SARS-CoV-2 and variants of concern after mRNA vaccines in patients with non-Hodgkin lymphoma and chronic lymphocytic leukemia. J Clin Oncol. 2022;40:3020–31.
Zeng C, Evans JP, Chakravarthy K, et al. COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 omicron variant in patients with cancer. Cancer Cell. 2022;40:117–9.
Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv. 2021;5:2624–43.
Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23:186–93.
Schioppo T, Ingegnoli F. Current perspective on rituximab in rheumatic diseases. Drug Des Devel Ther. 2017;11:2891–904.
Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007;26:3603–13.
Helfgott DC, Racine-Brzostek S, Short KJ, et al. Immunogenicity of COVID-19 mRNA vaccines in patients with acute myeloid leukemia and myelodysplastic syndrome. Leuk Lymphoma. 2023;64:662–70.
Narasimhan M, Mahimainathan L, Clark AE, et al. Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines. Vaccines (Basel). 2021;9:708.
Hammer H, Hoepner R, Friedli C, et al. Comparison of mRNA vaccinations with BNT162b2 or mRNA-1273 in anti-CD20-treated multiple sclerosis patients. Vaccines (Basel). 2022;10:922.
Macrae K, Martinez-Cajas J, Bessai K, Abdulhamed A, Gong Y. Quantitative analysis of SARS-CoV-2 antibody levels in cancer patients post three doses of immunization and prior to breakthrough COVID-19 infections. Curr Oncol. 2022;29:7059–71.
Verstappen GM, de Wolff L, Arends S, et al. Immunogenicity and safety of COVID-19 vaccination in patients with primary Sjögren's syndrome. RMD Open. 2022;8:e002265.
Su E, Fischer S, Demmer-Steingruber R, et al. Humoral and cellular responses to mRNA-based COVID-19 booster vaccinations in patients with solid neoplasms under active treatment. ESMO Open. 2022;7:100587.
Mairhofer M, Kausche L, Kaltenbrunner S, et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;39:1171–2.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
The authors thank Ekkehard Beck of Moderna, Inc., for critical review of the manuscript. Writing assistance was provided by Erin McClure, PhD, an employee of ICON (Blue Bell, PA, USA) in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors.
Funding
This study, including the journal’s Rapid Service fee, was funded by Moderna, Inc. Authors employed by Moderna, Inc., were involved in the study design, analysis and interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Sushma Kavikondala, Katrin Haeussler, and Xuan Wang designed and performed the systematic literature review and meta-analysis, interpreted data, and critically evaluated the manuscript. Anne Spellman and Anna Krivelyova designed and performed the systematic literature review and critically evaluated the manuscript. Pawana Sharma, Mohammadreza Amiri, Sonam Vats, Maria Nassim, and Nitendra Kumar collected data and critically evaluated the manuscript. Mary T. Bausch-Jurken and Nicolas Van de Velde conceptualized the article and provided oversight and critical evaluation of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
Xuan Wang, Pawana Sharma, Mohammadreza Amiri, Anna Krivelyova, Sonam Vats, Maria Nassim, and Nitendra Kumar are employees of ICON plc, a clinical research organization paid by Moderna, Inc., to conduct the study. Sushma Kavikondala and Katrin Haeussler are former employees of ICON plc. Anne Spellman is an independent epidemiology consultant/director of Data Health Ltd, which provides health data consultancy services, and was paid by Moderna, Inc., to conduct aspects of this study. Mary T. Bausch-Jurken and Nicolas Van de Velde are employees of Moderna, Inc., and hold stock/stock options in the company.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kavikondala, S., Haeussler, K., Wang, X. et al. Immunogenicity of mRNA-1273 and BNT162b2 in Immunocompromised Patients: Systematic Review and Meta-analysis Using GRADE. Infect Dis Ther 13, 1419–1438 (2024). https://doi.org/10.1007/s40121-024-00987-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00987-2