Abstract
Introduction
Dolutegravir/lamivudine (DTG/3TC) and dolutegravir/rilpivirine (DTG/RPV) are fixed-dose, complete, single-tablet, two-drug regimens (2DRs) indicated for HIV-1. DTG/3TC is approved for antiretroviral therapy (ART)-naive people with HIV-1 and virologically suppressed individuals to replace current ART; DTG/RPV is indicated for virologically suppressed individuals as a switch option. Virologic efficacy and effectiveness of these DTG-based 2DRs have been demonstrated in phase 3 clinical trials and real-world cohorts, primarily from Europe. This study characterized real-world use of DTG-based 2DRs for HIV-1 treatment in the USA.
Methods
TANDEM was a retrospective medical chart review across 24 US sites. Individuals aged ≥ 18 years who initiated DTG/3TC or DTG/RPV before September 30, 2020, with ≥ 6 months of follow-up were included. One cohort included ART-naive people who initiated DTG/3TC (n = 126), and two other cohorts included virologically suppressed (HIV-1 RNA < 50 copies/mL) people on stable ART regimens for ≥ 3 months before switch to either DTG/3TC (n = 192) or DTG/RPV (n = 151). Clinical characteristics, treatment history, and outcomes are described.
Results
Virologically suppressed individuals were older than those who were ART-naive, and the ART-naive cohort had higher proportions of individuals assigned male at birth and of Hispanic ethnicity. The most common healthcare provider-reported reason for choosing a DTG-based 2DR was avoidance of long-term toxicities (25–33% across cohorts), followed by simplification/streamlining of treatment. Among ART-naive people on DTG/3TC, 94% achieved virologic suppression after initiation, and 83% maintained suppression at last follow-up; discontinuation rate was < 1%. Among cohorts who switched to DTG-based 2DRs, 96% maintained virologic suppression on DTG/3TC and 93% on DTG/RPV; 2% on DTG/3TC and 3% on DTG/RPV discontinued.
Conclusion
Motivation for selecting DTG-based 2DRs was primarily driven by a desire to avoid or manage toxicities and simplify treatment. Results demonstrate that DTG/3TC and DTG/RPV are effective in real-world settings, with few discontinuations, reflecting data from clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Virologic efficacy of two-drug regimens (2DRs) dolutegravir/lamivudine (DTG/3TC) and dolutegravir/rilpivirine (DTG/RPV) for treatment of people with HIV-1 has been demonstrated in clinical trials, but limited evidence of effectiveness is available in USA-based real-world clinical settings. |
This study characterized clinical characteristics, treatment patterns, and outcomes with real-world use of DTG-based single-tablet 2DRs for the treatment of HIV-1. |
What was learned from the study? |
The most common reason for choosing a DTG-based 2DR reported by healthcare providers was avoidance of long-term toxicities (25–33% across cohorts), followed by simplification/streamlining of treatment, managing existing toxicity/intolerance, and patient preference. |
Among people who were antiretroviral therapy-naive and received DTG/3TC, 94% achieved virologic suppression after initiation, and 83% maintained virologic suppression at last follow-up; among virologically suppressed people who switched to a 2DR, 96% maintained suppression with DTG/3TC, and 93% maintained suppression with DTG/RPV. |
DTG/3TC and DTG/RPV are effective in real-world settings, with few treatment discontinuations, supporting their continued use in people with HIV-1 in the USA. |
Introduction
Advancements in the treatment of HIV-1 have made it possible to reduce the number of agents in daily antiretroviral therapy (ART) regimens to integrase strand transfer inhibitor (INSTI)-based two-drug regimens (2DRs) [1,2,3]. For people with HIV-1 who require lifelong therapy, use of 2DRs has the potential to reduce drug–drug interactions, long-term toxicities, and costs associated with multiple antiretroviral agents [1, 4].
In 2017, dolutegravir/rilpivirine (DTG/RPV) was the first complete single-tablet 2DR approved by the US Food and Drug Administration (FDA) for maintenance of virologic suppression (HIV-1 RNA < 50 copies/mL) [5, 6]. In the phase 3 SWORD-1 and SWORD-2 trials, DTG/RPV was non-inferior to three- or four-drug ART regimens through 48 weeks and maintained virologic suppression in 84% of participants through 3 years [7,8,9]. Long-term treatment with DTG/RPV was associated with a low virologic failure rate and favorable safety profile [9].
The second complete single-tablet 2DR, dolutegravir/lamivudine (DTG/3TC), was approved by the FDA in 2019 for the treatment of ART-naive individuals and in 2020 as a switch option for people who are virologically suppressed (HIV-1 RNA < 50 copies/mL) with no prior virologic failure and no history of resistance to either 3TC or DTG [10, 11]. In ART-naive adults, DTG + 3TC was non-inferior to DTG + tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), with 82% of participants virologically suppressed through 3 years in the double-blind, phase 3 GEMINI-1 and GEMINI-2 trials [12,13,14].
Two additional large studies investigated outcomes in virologically suppressed adults who switched to DTG/3TC [15,16,17,18]. In the phase 3 TANGO study, DTG/3TC was non-inferior to continuing three- or four-drug tenofovir alafenamide (TAF)-based regimens through 3 years, with durable efficacy, favorable safety and tolerability, and among those treated with DTG/3TC for 196 weeks, no development of drug resistance was observed through 4 years [15,16,17]. Similarly, in the phase 3 SALSA study, DTG/3TC was non-inferior to continuing a variety of three- or four-drug ART regimens through 48 weeks [18].
Real-world data for DTG/RPV and DTG/3TC support the results from clinical trials, with multiple studies demonstrating high rates of virologic suppression and favorable tolerability among ART-naive (DTG/3TC) and virologically suppressed individuals (DTG/RPV and DTG/3TC) [19,20,21,22,23,24]. However, the majority of data is derived from European cohorts, and there is limited evidence regarding treatment with these regimens in USA-based real-world clinical settings.
To better characterize real-world clinical experience with DTG-based single-tablet 2DRs, the TANDEM study was conducted to describe clinical characteristics and demographics and analyze evolving treatment patterns since the introduction and US Department of Health and Human Services guideline endorsement of DTG/3TC and DTG/RPV as 2DR options in the USA. Specifically, this study aimed to understand the characteristics of people with HIV-1 who are prescribed DTG-based 2DRs in real-world settings, to explore healthcare providers’ (HCPs’) primary motivation for initiating or switching individuals to DTG/3TC or DTG/RPV, and to further inform decision-making by providing clinical outcomes after initiation of DTG-based 2DRs in routine clinical practice.
Methods
Study Design
TANDEM was a USA-based, retrospective chart review study. Independent central institutional review board (IRB) ethical approval was granted by the Western IRB-Copernicus Group (WCG™ IRB, Princeton, NJ) on February 19, 2021 (reference number 20210451). Subsequent ethics reviews were provided by WCG IRB for each site before initiation of data collection.
Data were collected from 24 sites in the USA for people with HIV-1 who initiated or switched to DTG/3TC or DTG/RPV in accordance with the FDA-labeled indications on or after the index dates (DTG/3TC, May 1, 2019; DTG/RPV, December 1, 2017) and before September 30, 2020 (Fig. 1). Index dates were based on FDA approval dates for each regimen. Clinical follow-up of at least 6 months after start of DTG/3TC or DTG/RPV was required and could include time after discontinuation of DTG/3TC or DTG/RPV.
Study design. TANDEM was a retrospective medical chart review across 24 US sites. Individuals aged ≥ 18 years who initiated DTG/3TC or DTG/RPV before September 30, 2020, with ≥ 6 months of follow-up were included. One cohort included ART-naive individuals who initiated DTG/3TC, and two other cohorts included virologically suppressed (HIV-1 RNA < 50 copies/mL) individuals on stable ART regimens for ≥ 3 months before switch to either DTG/3TC or DTG/RPV. ART antiretroviral therapy, DTG dolutegravir, RPV rilpivirine, 3TC lamivudine
If individuals had a history of both DTG/3TC and DTG/RPV use, the most recent regimen with at least 6 months of clinical follow-up was included for evaluation. Individuals were not duplicated across study cohorts (i.e., each person included in the overall study was unique).
Selection of Study Population
To limit selection bias and include individuals with a range of treatment histories and follow-up times, sites abstracted data for half of their recruitment target by identifying the next consecutive “n” eligible person, working forward from the index date (Fig. 1). The remaining half of the recruitment target was identified by consecutively working backward from the cutoff date.
Inclusion criteria were age ≥ 18 years, diagnosis of HIV-1, and history of ART consisting of DTG/3TC or DTG/RPV as a single-tablet regimen. Upon initiation of DTG/3TC, individuals must have been either naive to ART or virologically suppressed on their current ART regimen; those who initiated DTG/RPV must have been virologically suppressed. “ART-naive” was defined as never having previously received any ART for treatment of HIV-1, although individuals could have received TDF/FTC or TAF/FTC as pre-exposure prophylaxis or any post-exposure prophylaxis before diagnosis of HIV-1. “Virologically suppressed” was defined as having HIV-1 RNA < 50 copies/mL while on a stable ART regimen for ≥ 3 months at time of switch to the DTG-based 2DR. Individuals with a history of prior virologic failure or history of prior resistance were included.
Exclusion criteria included use of ART without an approved HIV-1 indication; use of DTG + 3TC or DTG + RPV as a multiple-tablet regimen before initiation of single-tablet DTG/3TC or DTG/RPV; and involvement in prior ViiV Healthcare-sponsored clinical trials including SALSA, STAT, SWORD-1/-2, GEMINI-1/-2, TANGO, or ACTG 5353.
Objectives and Measures
Primary objectives of this analysis were to describe the demographics and clinical characteristics of people with HIV-1 who initiated or switched to DTG/3TC or DTG/RPV and the clinical rationale for initiating or switching to DTG/3TC or DTG/RPV. Secondary objectives were to describe virologic outcomes, rates of discontinuation, and reasons for discontinuing DTG/3TC or DTG/RPV.
Clinical characteristics, treatment history, and outcomes after initiation of, or switch to, DTG-based 2DRs were abstracted by clinic staff from medical records through the completion of an electronic case report form, comprising multiple-choice or open-text fields with limited characters. When the data were not explicitly included in the medical record, the principal investigator was asked to provide further information about the clinical rationale or primary reason for initiating or switching to DTG/3TC or DTG/RPV based on knowledge of the case.
To fulfill the primary study objectives, HCPs or clinic staff extracted demographic and clinical characteristics as available, including age, sex assigned at birth, current gender identity, race and ethnicity, type of insurance coverage, time since first ART initiation, number of prior ART regimens and time on therapy, time on DTG/3TC or DTG/RPV, HIV-1 RNA at time of ART initiation and before DTG/3TC or DTG/RPV initiation, CD4+ cell count at time of ART initiation and before DTG/3TC or DTG/RPV initiation, and drug resistance profiles, if performed. Clinic staff also indicated whether ART-naive individuals received DTG/3TC as part of a test-and-treat paradigm, in which treatment was initiated shortly after HIV-1 diagnosis and in the absence of known laboratory values for viral load, CD4+ cell count, and HIV-1 resistance mutations. Treatment considerations currently or previously relevant to each person were selected from a list. The primary reason for initiating or switching to DTG/3TC or DTG/RPV was selected from a pre-defined drop-down list and could be inferred by the HCP on the basis of knowledge of the case. HCPs were also asked about the impact of COVID-19 on the decision to initiate or switch to DTG/3TC or DTG/RPV.
To fulfill the secondary objectives, HCPs were asked whether ART-naive individuals achieved virologic suppression (HIV-1 RNA < 50 copies/mL) after starting DTG/3TC and to report time from DTG/3TC initiation to virologic suppression. HCPs also reported whether ART-naive individuals on DTG/3TC experienced virologic rebound (two consecutive measurements of HIV-1 RNA ≥ 200 copies/mL after achieving suppression to < 50 copies/mL) as well as time from virologic suppression to rebound. For virologically suppressed cohorts, HCPs were asked whether the individual maintained HIV-1 RNA < 50 copies/mL after switch to DTG/3TC or DTG/RPV and to report time from start of the DTG-based 2DR to becoming virologically detectable.
For all cohorts, HCPs reported whether the individual discontinued their most recent DTG/3TC or DTG/RPV regimen, time from initiation to discontinuation, and primary reason for discontinuation.
Statistical Analysis
The 24 participating sites were each asked to abstract data for at least 15 and no more than 25 individuals (approximately 5% of the total study population per site) to ensure a representative sample.
Analyses were descriptive, and no formal hypothesis testing was conducted. Descriptive statistics included percentages, mean (standard deviation [SD]), and median (interquartile range [IQR]; first and third quartiles). Missing data were not imputed. Descriptive analyses were performed using IBM® SPSS® Data Collection Survey Reporter software (version 7.5; IBM, Armonk, NY). Time-to-event findings were described using Kaplan–Meier charts to visually estimate the distribution of times. Time-to-event outcomes were calculated using Kaplan–Meier estimators conducted in StataCorp 2015, Stata Statistical Software: Release 16 (StataCorp LLC, College Station, TX).
Results
Disposition, Demographics, and Clinical Characteristics
The number of people with HIV-1 abstracted by each study site ranged from 4 to 25, and all but three sites abstracted at least 17 records. Of 469 individuals included in the study, 126 (27%) were naive to ART before initiation of DTG/3TC, 61 (48%) of whom received DTG/3TC as part of a test-and-treat paradigm. The remaining individuals (n = 343, 73%) were ART-experienced and virologically suppressed. Of these, 192 (41%) switched to DTG/3TC and 151 (32%) switched to DTG/RPV. Individuals in the virologically suppressed cohorts were older than those who were ART-naive, and the ART-naive cohort had higher proportions of individuals assigned male at birth, of Hispanic ethnicity, and enrolled in the AIDS Drug Assistance Program (Table 1).
Among those receiving DTG-based 2DRs at data cutoff, median time on regimen was 65.3 weeks (1.3 years) for ART-naive people receiving DTG/3TC, 81.1 weeks (1.6 years) for virologically suppressed people receiving DTG/3TC, and 143.3 weeks (2.8 years) for people receiving DTG/RPV (Table 2; DTG/RPV and DTG/3TC were approved as complete ART regimens by the FDA in 2017 and 2019, respectively). Before initiation of a DTG-based 2DR, virologically suppressed individuals had spent a median of 447.6–473.3 weeks (> 8 years) on prior ART regimens (Table 2, Table S1). Dolutegravir/abacavir/lamivudine was the most common ART regimen received immediately before DTG/3TC (27%) or DTG/RPV (17%) in virologically suppressed cohorts (Table S1).
In the ART-naive cohort (N = 126), drug resistance testing detected no resistance in 63 (50%) individuals and resistance in 20 (16%); resistance testing was not performed for 35 (28%) individuals (Table 3). In this cohort, 16 (13%) had resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs; n = 6 with K103N/S), 7 (6%) had resistance to protease inhibitors (PIs), and 1 (< 1%) each had resistance to nucleoside reverse transcriptase inhibitors (NRTIs) or INSTIs; some had resistance to more than one class.
In the virologically suppressed cohorts, no drug resistance testing was performed at switch for 170 (89%) of 192 individuals receiving DTG/3TC and 111 (74%) of 151 receiving DTG/RPV (Table 3). Resistance testing was performed at switch for 13 (7%) virologically suppressed individuals receiving DTG/3TC and resistance was detected in 4 (2%); 3 (2%) had resistance to NRTIs (n = 2 with M184V/I), and 1 (< 1%) each had resistance to NNRTIs and PIs. Among virologically suppressed individuals receiving DTG/RPV, resistance testing was performed at switch for 31 (21%) and resistance was detected in 16 (11%); 15 (10%) had resistance to NRTIs (n = 10 with M184V/I), 6 (4%) each had resistance to NNRTIs (n = 4 with K103N/S) and PIs, and 1 (< 1%) had resistance to INSTIs.
Treatment Considerations
For the ART-naive cohort, the most common treatment considerations cited by HCPs were limited access to healthcare, comorbidities, mental health issues, health insurance issues, job instability, and low health literacy (Table 4). For the virologically suppressed cohorts, the most common treatment considerations were comorbidities and polypharmacy, followed by health insurance issues and mental health issues (Table 4).
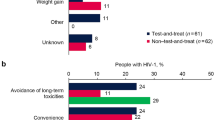
Across all cohorts, the primary HCP-reported reason for initiating DTG-based single-tablet 2DRs was avoidance of long-term toxicities (reported for ≥ 25% of individuals in each cohort; Fig. 2). For those naive to ART, the next most common primary reasons for initiating DTG/3TC were convenience, patient preference, simplification/streamlining of treatment, and weight gain. For those who were virologically suppressed, the next most common reasons for switching to DTG-based 2DRs were simplification/streamlining of treatment and managing existing toxicity/intolerance issues (Fig. 2).
For one person, the HCP reported that considerations related to the COVID-19 pandemic resulted in selection of a DTG-based 2DR.
Virologic Outcomes
HCPs reported that 118 (94%) of 126 ART-naive individuals achieved virologic suppression (HIV-1 RNA < 50 copies/mL) after DTG/3TC initiation, and 105 (83% of the full sample) remained suppressed at last follow-up (Fig. 3a). A median (IQR) of 10.4 (5.7, 19.1) weeks elapsed between DTG/3TC initiation and virologic suppression (Fig. S1A). For the 6 (5%) people who experienced virologic rebound (two consecutive HIV-1 RNA measurements ≥ 200 copies/mL after suppression to < 50 copies/mL), median (IQR) time from virologic suppression to rebound was 20.9 (17.3, 81.4) weeks (Fig. S1B); only one of six who rebounded started DTG/3TC in a test-and-treat setting. Of the 3 (2%) who remained virologically detectable after DTG/3TC initiation, all were enrolled under a test-and-treat paradigm.
Virologic outcomes after a initiation of DTG/3TC in ART-naive individuals or b switch to DTG/3TC or DTG/RPV in virologically suppressed individuals. Virologic suppression defined as HIV-1 RNA < 50 copies/mL. Virologic rebound defined as two consecutive measurements of HIV-1 RNA ≥ 200 copies/mL after achieving suppression to < 50 copies/mL. ART antiretroviral therapy; DTG dolutegravir; RPV rilpivirine; 3TC lamivudine
Among virologically suppressed individuals, 184 (96%) of 192 who switched to DTG/3TC and 141 (93%) of 151 who switched to DTG/RPV maintained virologic suppression (Fig. 3b). Seven (4%) people who switched to DTG/3TC and 9 (6%) who switched to DTG/RPV subsequently became virologically detectable; of these, 4 (2%) who switched to DTG/3TC and 4 (3%) who switched to DTG/RPV remained on their DTG-based regimen and resuppressed. Median (IQR) time from initiation of DTG-based 2DR to becoming virologically detectable was 29.4 (4.4, 96.0) weeks for the DTG/3TC group and 62.0 (48.0, 71.0) weeks for the DTG/RPV group (Fig. S2).
Three individuals who switched to DTG/3TC had NRTI resistance (two with archived M184V/I mutations) at baseline; all remained suppressed during the study period.
Discontinuations
Four individuals had discontinued DTG/3TC as of the data cutoff: 1 (< 1%) of 126 in the ART-naive cohort discontinued after 60.9 weeks because of viremia (persistent low-level viremia or viral blips), and 3 (2%) of 192 in the virologically suppressed cohort discontinued after a median (IQR) of 29.6 (21.6, 74.3) weeks (Fig. S3); one person each discontinued because of toxicity/intolerance, concerns about weight gain, and patient preference. Resistance testing was performed for two of the four individuals, and no resistance was detected.
Among virologically suppressed individuals who switched to DTG/RPV (N = 151), as of the data cutoff, 4 (3%) discontinued after a median (IQR) of 120.1 (85.2, 161.4) weeks: two because of regimen-related food requirements, one because of virologic failure (most likely due to non-adherence to ART), and one because of patient preference. Resistance testing was performed for one of four people who discontinued DTG/RPV, and an NNRTI resistance mutation (K101P/E/H and other mutation not specified) was detected in this individual.
Discussion
In the TANDEM study, individuals in the virologically suppressed cohorts were older than those in the ART-naive cohort, and the ART-naive cohort had higher proportions of individuals assigned male at birth and of Hispanic ethnicity. The most common HCP-reported reason to initiate or switch to a DTG-based single-tablet 2DR (in both ART-naive and virologically suppressed cohorts) was avoidance of long-term toxicities. Among ART-naive individuals who received DTG/3TC, 94% achieved virologic suppression after initiation, and 83% maintained suppression at last follow-up. Few discontinuations of DTG-based 2DRs occurred. These real-world data support results from clinical trials demonstrating that DTG-based single-tablet 2DRs are effective for achieving virologic suppression in ART-naive people with HIV-1 and for maintaining virologic suppression in people switching from stable ART regimens.
In TANDEM, 94% of treatment-naive individuals achieved virologic suppression after initiating DTG/3TC, and 83% maintained suppression at last follow-up with a median follow-up of 65 weeks. In the pooled analysis of the phase 3 GEMINI-1 and GEMINI-2 trials, the proportion of participants who maintained virologic suppression was 91% at 48 weeks and 86% at 96 weeks [12, 13]. Compared with real-world observations in ART-naive individuals initiating DTG + 3TC or DTG/3TC fixed-dose combination in the REDOLA cohort, the proportion maintaining virologic suppression in TANDEM was generally consistent (85% at 48 weeks and 84% at 96 weeks) [19, 25].
Only 6 (5%) of 126 ART-naive people in TANDEM experienced viral rebound after achieving virologic suppression, and only 1 (< 1%) discontinued DTG/3TC by data cutoff; 12 (10%) had unknown virologic status or were lost to follow-up, likely contributing to the lower rates of virologic suppression observed at last follow-up.
In people who were virologically suppressed on their previous regimen before switching to a DTG-based single-tablet 2DR, the proportion who maintained undetectable viral load at follow-up was very high (96% at median 81-week follow-up with DTG/3TC and 93% at median 143-week follow-up with DTG/RPV). For DTG/3TC, results from TANDEM were consistent with those from the phase 3 TANGO and SALSA studies, in which 93% of participants who switched to DTG/3TC maintained virologic suppression at 48 weeks [15], 86% at 96 weeks [16], and 86% at 144 weeks in TANGO [16] and 94% maintained virologic suppression at 48 weeks in SALSA [18]. Results from TANDEM are also consistent with a 2021 systematic review, which reported that the proportion of people who switched to DTG + 3TC or DTG/3TC fixed-dose combination and maintained virologic suppression at 96 weeks of follow-up ranged from 92% to 100% in studies with at least 100 individuals with known outcomes [22]. In TANDEM, virologic suppression was maintained throughout the study period in the two individuals with archived M184V/I at baseline who switched to DTG/3TC; these results are consistent with those from TANGO, in which virologic suppression was maintained through 144 weeks in all four participants who switched to DTG/3TC with baseline archived M184V/I [9, 16]. Results for DTG/RPV from TANDEM were also supportive of clinical trial observations: in the pooled analysis of the SWORD-1 and SWORD-2 trials in which virologically suppressed participants switched to DTG/RPV, 95% maintained virologic suppression at 48 weeks after switch, 89% at 96 weeks, and 84% at 148 weeks [9].
According to treating HCPs, the most common primary reason for initiating a DTG-based single-tablet 2DR (in all study cohorts) was avoidance of long-term toxicities. Other reasons commonly given for initiating DTG-based 2DRs included simplification/streamlining of treatment, managing existing toxicity/intolerance issues, patient preference, convenience, and weight gain. The TANDEM study included individuals with treatment considerations such as comorbidities, polypharmacy, mental health issues, health insurance issues, and limited access to healthcare. Thus, this study demonstrates that DTG-based singlet-tablet 2DRs can effectively meet the varied needs of people with HIV-1 in real-world settings as they implement their personal goals for healthy living [26].
One study limitation was that TANDEM was reliant on clinic staff being willing and having sufficient resources to participate in the study. Sites were approached and selected for their ability to enroll enough people who had previously received DTG/3TC or DTG/RPV; therefore, the treatment practices at these centers may not be representative of all HIV treatment centers across the USA. Additionally, over 75% of the included population across study cohorts was male, which is consistent with the proportion of newly diagnosed individuals in the USA in 2021 (79% were male sex at birth), but nonetheless TANDEM’s results may limit generalizability to individuals of other genders [27]. As resistance testing was not performed for the majority of people in the virologically suppressed cohorts, data on switching to DTG/3TC or DTG/RPV among individuals with baseline resistance mutations were limited. Lastly, some data may have been subject to HCP recall.
Conclusion
Overall, observations from the TANDEM study demonstrate that DTG-based single-tablet 2DRs are often selected in real-world practice to avoid long-term toxicities. DTG/3TC and DTG/RPV are effective for the treatment of HIV-1 in real-world settings in the USA and rarely discontinued.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy reasons. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
References
Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs. 2017;7:113–4.
Katlama C, Ghosn J, Murphy RL. Individualized antiretroviral therapeutic approaches: less can be more. AIDS. 2017;31:1065–71.
Cento V, Perno CF. Two-drug regimens with dolutegravir plus rilpivirine or lamivudine in HIV-1 treatment-naive, virologically-suppressed patients: latest evidence from the literature on their efficacy and safety. J Glob Antimicrob Resist. 2020;20:228–37.
Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis. 2016;62:784–91.
Juluca [prescribing information]. Durham: ViiV Healthcare; 2022.
US Food and Drug Administration. FDA approves first two-drug regimen for certain patients with HIV. 21 Nov 2017, https://www.fda.gov/news-events/press-announcements/fda-approves-first-two-drug-regimen-certain-patients-hiv. Accessed 14 Dec 2023.
Llibre JM, Hung C-C, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839–49.
Aboud M, Orkin C, Podzamczer D, et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV. 2019;6:e576–87.
van Wyk J, Orkin C, Rubio R, et al. Brief report: durable suppression and low rate of virologic failure 3 years after switch to dolutegravir + rilpivirine 2-drug regimen: 148-week results from the SWORD-1 and SWORD-2 randomized clinical trials. J Acquir Immune Defic Syndr. 2020;85:325–30.
Dovato [prescribing information]. Durham: ViiV Healthcare; 2023.
US Food and Drug Administration. FDA approves first two-drug complete regimen for HIV-infected patients who have never received antiretroviral treatment. 8 Apr 2019, https://www.fda.gov/news-events/press-announcements/fda-approves-first-two-drug-complete-regimen-hiv-infected-patients-who-have-never-received. Accessed 14 Dec 2023.
Cahn P, Sierra Madero J, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393:143–55.
Cahn P, Sierra Madero J, Arribas JR, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr. 2020;83:310–8.
Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy-naive adults with HIV-1 infection. AIDS. 2022;36:39–48.
van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis. 2020;71:1920–9.
Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis. 2022;75:975–86.
De Wit S, Bonnet F, Osiyemi O, et al. Durable efficacy of switching from a 3- or 4-drug tenofovir alafenamide–based regimen to the 2-drug regimen dolutegravir/lamivudine in the TANGO study through week 196. J Acquir Immune Defic Syndr. 2024. https://doi.org/10.1097/QAI.0000000000003395.
Llibre JM, Brites C, Cheng C-Y, et al. Efficacy and safety of switching to the 2-drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with human immunodeficiency virus 1 (HIV-1): week 48 results from the phase 3, noninferiority SALSA randomized trial. Clin Infect Dis. 2023;76:720–9.
Cabello-Ubeda A, López Bernaldo de Quirós JC, Carbonero LM, et al. 48-Week effectiveness and tolerability of dolutegravir (DTG) + lamivudine (3TC) in antiretroviral-naive adults living with HIV: a multicenter real-life cohort. PLoS ONE. 2022;17:e0277606.
Hidalgo-Tenorio C, Pasquau J, Vinuesa D, et al. DOLAVI real-life study of dolutegravir plus lamivudine in naive HIV-1 patients (48 weeks). Viruses. 2022;14:524.
Dueñas-Gutiérrez C, Buzón L, Pedrero-Tomé R, et al. Efficacy and safety of two-drug regimens with dolutegravir plus rilpivirine or lamivudine in HIV-1 virologically suppressed people living with HIV. Viruses. 2023;15:936.
Patel R, Evitt L, Mariolis I, et al. HIV treatment with the two-drug regimen dolutegravir plus lamivudine in real-world clinical practice: a systematic literature review. Infect Dis Ther. 2021;10:2051–70.
Schabaz F, Scherzer J, Schneeweiß S, et al. 3-year outcomes of dolutegravir/rilpivirine in virologically suppressed HIV-infected PLHIV: real-world data from the prospective German JUNGLE cohort. Presented at: HIV Drug Therapy Glasgow 2022; October 23–26, 2022; Glasgow, Scotland.
Noe S, Scholten S, Wyen C, et al. 3-year outcomes for dolutegravir (DTG) + lamivudine (3TC) in ART-naive and pre-treated people living with HIV-1 (PLHIV) in Germany: real-world data from the German URBAN cohort. Presented at: 19th European AIDS Conference; October 18–21, 2023; Warsaw, Poland.
Pulido F, López Bernaldo de Quirós JC, Górgolas M, et al. 96 weeks effectiveness and tolerability of DTG + 3TC in naive patients: the REDOLA study. Presented at: HIV Drug Therapy Glasgow 2022; October 23–26, 2022; Glasgow, Scotland.
Guaraldi G, Arends J, Buhk T, et al. “Moving fourth”: a vision toward achieving healthy living with HIV beyond viral suppression. AIDS Rev. 2019;21:135–42.
Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas, 2021. HIV Surveillance Report, 2021; vol. 34. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2023. Accessed 14 Dec 2023.
Acknowledgements
The authors would like to thank the study investigators across each of the 24 USA-based sites for their contributions toward the study in abstracting the data from medical charts of individuals who either initiated or switched to DTG/3TC or DTG/RPV. Data included in this manuscript have previously been presented in part at the 24th International AIDS Conference; July 29–August 2, 2022; Virtual and Montreal, Canada; Poster EPB147 and the Academy of Managed Care Pharmacy Nexus; October 11–14, 2022; National Harbor, MD; Poster.
Medical Writing, Editorial and Other Assistance
Editorial assistance was provided under the direction of the authors by Lisa Baker, PhD, CMPP, and Jennifer Rossi, MA, ELS, MedThink SciCom, and funded by ViiV Healthcare.
Funding
This study was funded by ViiV Healthcare (Durham, NC, USA). The journal’s Rapid Service fee was funded by ViiV Healthcare.
Author information
Authors and Affiliations
Contributions
Cynthia Donovan, Gavin Harper, Deanna Merrill, Aimee A. Metzner, Katie Mycock, Hannah Wallis, Jimena Patarroyo, and Alan Oglesby contributed to the conception of the study. Stefan Schneider, Gary Blick, Christina Burke, Douglas Ward, Paul Benson, Franco Felizarta, Dallas Green, Cynthia Donovan, Gavin Harper, Deanna Merrill, Aimee A. Metzner, Katie Mycock, Hannah Wallis, Jimena Patarroyo, and Alan Oglesby contributed to the design of the study and the acquisition of data. Cynthia Donovan, Gavin Harper, Deanna Merrill, Aimee A. Metzner, Katie Mycock, Hannah Wallis, Jimena Patarroyo, Andrew P. Brogan, and Alan Oglesby contributed to the analysis and interpretation of data. Cynthia Donovan, Aimee A. Metzner, and Andrew P. Brogan contributed to drafting the manuscript. All authors contributed to critically revising the manuscript for important intellectual content and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of Interest
Stefan Schneider has received honoraria from GSK. Gary Blick has received honoraria from ViiV Healthcare for participation in speakers bureaus and advisory boards. Christina Burke has received study support from Adelphi Real World and ViiV Healthcare, paid to her institution. Douglas Ward has no conflicts to report. Paul Benson has received honoraria from ViiV Healthcare. Franco Felizarta has received grants and honoraria from AbbVie, Gilead, and ViiV Healthcare. Dallas Green has served as a principal investigator or sub-investigator in clinical research trials sponsored by GSK and ViiV Healthcare and participated in speakers bureaus and advisory boards for GSK and ViiV Healthcare, and is a voting member of the Miami-Dade HIV/AIDS Partnership medical sub-committee. Cynthia Donovan, Deanna Merrill, Aimee A. Metzner, Jimena Patarroyo, Andrew P. Brogan, and Alan Oglesby are employees of ViiV Healthcare and may own stock in GSK. Gavin Harper, Katie Mycock, and Hannah Wallis are employees of Adelphi Real World, which was contracted by ViiV Healthcare for this study.
Ethical Approval
Independent central institutional review board (IRB) ethical approval was granted by the Western IRB-Copernicus Group (WCG™ IRB, Princeton, NJ) on February 19, 2021 (reference number, 20210451). Subsequent ethics reviews were provided by WCG IRB for each site before initiation of data collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Schneider, S., Blick, G., Burke, C. et al. Two-Drug Regimens Dolutegravir/Lamivudine and Dolutegravir/Rilpivirine Are Effective with Few Discontinuations in US Real-World Settings: Results from the TANDEM Study. Infect Dis Ther 13, 891–906 (2024). https://doi.org/10.1007/s40121-024-00961-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00961-y