Abstract
Introduction
Dolutegravir/lamivudine (DTG/3TC) was first approved by the US Food and Drug Administration in 2019 for the treatment of antiretroviral therapy (ART)-naive people with HIV-1 based on results from the pivotal GEMINI-1/GEMINI-2 trials. Around that time, immediate initiation of treatment upon diagnosis was recommended in the US Department of Health and Human Services guidelines. Here we report results from 126 treatment-naive people with HIV-1 who initiated DTG/3TC as part of a test-and-treat strategy (n = 61) or with high baseline viral loads (HIV-1 RNA ≥ 100,000 copies/ml; n = 16) from the TANDEM study.
Methods
TANDEM was a US-based, retrospective chart review study that included a cohort of 126 individuals aged ≥ 18 years with no prior history of ART who initiated DTG/3TC before September 30, 2020, and had ≥ 6 months of follow-up. Test-and-treat was defined as ART initiation shortly after diagnosis without available viral load, CD4 + cell count, or HIV-1 resistance data. Outcomes included virologic suppression (HIV-1 RNA < 50 copies/ml; overall and by baseline viral load) and discontinuations. Analyses were descriptive.
Results
Among 61 individuals who initiated DTG/3TC in a test-and-treat setting (median [interquartile range (IQR)] treatment duration, 1.3 [0.9–1.7] years), 57 (93%) achieved virologic suppression, and 51 (84%) remained suppressed; 1 (< 1%) individual discontinued DTG/3TC due to persistent low-level viremia. The most common healthcare provider (HCP)-reported reason for initiating DTG/3TC was avoidance of long-term toxicities among individuals in the test-and-treat subgroup. Of 16 treatment-naive individuals with high baseline viral loads (median [IQR] treatment duration, 100,000–250,000 copies/ml: 1.2 [0.8–1.8] years; > 250,000 copies/ml: 1.0 [0.7–1.1] years), 14 (88%) achieved virologic suppression, 13 (81%) remained suppressed, and none discontinued DTG/3TC. Patient preference was the most common HCP-reported reason for initiating DTG/3TC in this subgroup.
Conclusions
Results demonstrate real-world effectiveness of DTG/3TC, with few discontinuations, in people with HIV-1 in test-and-treat settings or with high baseline viral loads.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Evidence for treatment-naive people with HIV-1 initiating antiretroviral therapy under a test-and-treat approach or with high baseline viral loads in real-world clinical settings is limited. |
This study describes outcomes with real-world use of dolutegravir/lamivudine (DTG/3TC) in treatment-naive individuals in the TANDEM study who either initiated as part of a test-and-treat strategy or with high baseline viral loads (≥ 100,000 copies/ml). |
What was learned from this study? |
Among 61 people with HIV-1 who initiated DTG/3TC in a test-and-treat setting, 57 (93%) achieved virologic suppression, 51 (84%) remained suppressed, and one discontinued treatment; among 16 people with HIV-1 and high baseline viral loads, 14 (88%) achieved virologic suppression, 13 (81%) remained suppressed, and none discontinued DTG/3TC. |
Real-world use of DTG/3TC was effective, with few discontinuations, in treatment-naive people with HIV-1 in test-and-treat settings and among those with high baseline viral loads, supporting results from clinical trials. |
Introduction
Since 2017, antiretroviral therapy (ART) has evolved to include single-tablet, integrase inhibitor-based, 2-drug regimens (2DRs) approved for treatment of people with HIV-1 [1,2,3]. These regimens have demonstrated non-inferior efficacy and good safety and tolerability profiles when compared with 3- or 4-drug regimens. By reducing the number of medicines people with HIV-1 take, there is the potential to reduce drug–drug interactions, long-term toxicities associated with multiple antiretrovirals, and costs [4,5,6,7]. Dolutegravir/lamivudine (DTG/3TC) is a single-tablet 2DR indicated as a complete regimen for the treatment of HIV-1 in adults with no history of ART or to replace the current antiretroviral regimen in those who are virologically suppressed [2].
The phase 3 GEMINI-1/-2 studies evaluated efficacy and safety of DTG + 3TC in a treatment-naive population [8,9,10]. In the pooled analysis of GEMINI-1/-2, 92% (129/140) of participants with baseline viral loads > 100,000 copies/ml, 88% (45/51) with baseline viral loads > 250,000 copies/ml, and 85% (11/13) with baseline viral loads > 500,000 copies/ml achieved virologic suppression (HIV-1 RNA < 50 copies/ml) after 48 weeks of DTG + 3TC, compared with 91% (526/576) of participants with baseline viral loads ≤ 100,000 copies/ml [11].
Initiation of ART soon after HIV diagnosis (ideally on the same day) and in the absence of clinical information such as viral load, CD4 + cell count, and HIV-1 resistance data, commonly referred to as test-and-treat, has been recommended by the US Department of Health and Human Services guidelines as a means to increase ART uptake and strengthen linkage to care [12]. Use of DTG/3TC as first-line therapy in a test-and-treat setting was investigated in the open-label, phase 3b STAT trial [13]. In this study, 82% (107/131) of participants in the intention-to-treat–exposed (ITT-E) missing = failure analysis achieved virologic suppression (HIV-1 RNA < 50 copies/ml) at week 48, regardless of ART [14]. Furthermore, 82% (42/51) of participants with baseline viral loads ≥ 100,000 copies/ml achieved virologic suppression at week 48 compared with 81% (64/79) with baseline viral loads < 100,000 copies/ml (ITT-E missing = failure analysis) [14].
Although these data are available for DTG/3TC in clinical trial settings, evidence for treatment-naive individuals initiating ART under a test-and-treat approach or with high baseline viral loads in real-world clinical settings is limited, particularly in the United States [11, 13]. In the US-based, retrospective TANDEM study, 94% (118/126) of treatment-naive individuals achieved virologic suppression after initiation of DTG/3TC, and 83% remained suppressed after a median of 1.3 years of treatment. Only one (< 1%) person discontinued DTG/3TC by data cutoff [15]. Outcomes for treatment-naive individuals in the TANDEM study who initiated DTG/3TC as part of a test-and-treat strategy or who initiated with high baseline viral loads (defined as 100,000–250,000 copies/ml and > 250,000 copies/ml) are described here.
Methods
Study Design
TANDEM was a US-based, retrospective chart review study. Independent central institutional review board (IRB) ethical approval was granted by the Western IRB-Copernicus Group (WCG™ IRB, Princeton, NJ) on February 19, 2021 (reference number, 20210451). Subsequent ethics reviews were provided by WCG IRB for each site before initiation of data collection.
Detailed methods for the TANDEM study have previously been reported [15]. Briefly, data were collected from 24 sites in the United States. TANDEM included people with HIV-1 who initiated or switched to DTG/3TC or DTG/rilpivirine in accordance with US Food and Drug Administration (FDA)-labeled indications. Findings are reported here for treatment-naive people who initiated DTG/3TC.
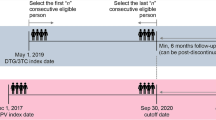
Eligible individuals were aged ≥ 18 years, had a diagnosis of HIV-1, and had been treated with single-tablet DTG/3TC with at least 6 months of follow-up, which could include time post-discontinuation. Treatment with DTG/3TC was initiated on or after May 1, 2019, and before September 30, 2020. “Treatment-naive” was defined as never having previously received any ART for treatment of HIV-1, although individuals could have received pre- or post-exposure prophylaxis before diagnosis of HIV-1.
Measures included demographics, baseline clinical characteristics, clinical rationale for initiating DTG/3TC, virologic outcomes after initiation, rates of discontinuation, and reasons for discontinuation. Virologic suppression was defined as HIV-1 RNA < 50 copies/ml, virologic detection was defined as HIV-1 RNA ≥ 50 copies/ml, and virologic rebound was defined as two consecutive measurements of HIV-1 RNA ≥ 200 copies/ml after achieving virologic suppression. Measures were reported from individual medical records by principal investigators and/or clinic staff.
Subgroup Analyses
Treatment-naive individuals from TANDEM who initiated DTG/3TC were stratified by those who started ART as part of a test-and-treat strategy and those who did not. In the test-and-treat subgroup, DTG/3TC was initiated shortly after HIV-1 diagnosis and in the absence of known laboratory values for viral load, CD4 + cell count, or HIV-1 resistance mutations.
In addition, the treatment-naive study group was also stratified by viral load at baseline (i.e., immediately before DTG/3TC initiation). High baseline viral load subgroups were defined as those with baseline viral loads of 100,000–250,000 copies/ml and > 250,000 copies/ml.
Baseline viral load values were not available for individuals in the test-and-treat subgroup; therefore, there is no overlap between the test-and-treat subgroup and the high baseline viral load subgroups.
Statistical Analysis
Analyses were descriptive, and no formal hypothesis testing was conducted. Descriptive statistics included percentages, mean (standard deviation [SD]), and median (interquartile range [IQR]; first and third quartiles). Missing data were not imputed. Descriptive analyses were performed using IBM® SPSS® Data Collection Survey Reporter software (version 7.5; IBM, Armonk, NY, USA).
Results
Disposition and Baseline Characteristics
From the overall TANDEM population of 469 people with HIV-1, 126 (27%) were treatment-naive, 58 of whom had baseline viral load data: 42 (33%) of 126 had baseline viral loads < 100,000 copies/ml and 16 (13%) of 126 had baseline viral loads ≥ 100,000 copies/ml. Of the 16 individuals with high baseline viral loads, 9 (56%) had HIV-1 RNA 100,000–250,000 copies/ml and 7 (44%) had HIV-1 RNA > 250,000 copies/ml (4 [25%] with HIV-1 RNA ≥ 500,000 copies/ml). Sixty-one (48%) of 126 treatment-naive individuals received DTG/3TC in a test-and-treat setting, 34 (56%) of whom started DTG/3TC on free samples. Demographic characteristics among the test-and-treat subgroup and high baseline viral load subgroups were similar to those of the overall treatment-naive population (Table 1).
For the test-and-treat subgroup, limited access to healthcare, mental health issues, and job instability were the most common relevant DTG/3TC treatment or prescribing considerations reported by healthcare providers (HCPs; Table 2), although across all subgroups, “no relevant treatment considerations identified” was the most frequent answer. Drug resistance testing was performed for 42 (69%) individuals in the test-and-treat subgroup, and resistance (primarily to non-nucleoside reverse transcriptase inhibitors and protease inhibitors) was detected in seven (11%) (Table 2).
As expected, individuals with high baseline viral loads had lower median CD4 + cell counts at baseline compared with the overall treatment-naive group, and median baseline CD4 + cell counts were lower with increasing baseline viral loads (Table 2). Comorbidities were reported by HCPs as a relevant DTG/3TC treatment or prescribing consideration for two of the 16 individuals with high baseline viral loads; health insurance issues or changes, low health literacy, substance abuse (i.e., injection drug use, alcohol abuse), affordability of HIV medications, and difficult work and/or family schedule were each reported for one individual with high baseline viral loads. Drug resistance testing was performed for seven (44%) individuals with high baseline viral loads, and resistance-associated mutations were detected in one (6%) person (Table 2).
Treatment and Virologic Outcomes: Test-and-Treat Subgroup
In the test-and-treat subgroup, avoidance of long-term toxicities was the primary HCP-reported reason for initiating DTG/3TC for 26 (43%) individuals, followed by simplification/streamlining of treatment (n = 8 [13%]) and convenience (n = 7 [11%]; Fig. 1B).
Healthcare provider–reported primary reason for using DTG/3TC by A initiation status in a test-and-treat setting and B baseline viral load. The primary reason for initiating DTG/3TC was selected from a list and could be inferred. Response options ordered by frequency in overall group of treatment-naive people with HIV-1 (data not shown) and reported for responses selected for > 1 person in the overall group. Only one option could be selected. DTG dolutegravir, 3TC lamivudine
Sixty (98%) individuals in the test-and-treat subgroup remained on DTG/3TC at data cutoff, for a median (IQR) treatment duration of 1.3 (0.9–1.7) years (Table 3). One individual discontinued DTG/3TC because of persistent low-level viremia or “viral blips.” No resistance was detected when resistance testing was conducted at time of discontinuation; no virologic outcomes were measured after DTG/3TC discontinuation. Among those who initiated DTG/3TC under a test-and-treat strategy, 57 (93%) achieved virologic suppression (HIV-1 RNA < 50 copies/ml) after a median of 9.7 weeks, 51 (84%) remained virologically suppressed at data cutoff, one (2%) experienced virologic rebound (two consecutive HIV-1 RNA measurements ≥ 200 copies/ml after suppression), and five (8%) were lost to follow-up (Table 3, Fig. 2B). In the test-and-treat subgroup, three (5%) people remained virologically detectable after DTG/3TC initiation through data cutoff.
Healthcare providers reported that 57 (93%) individuals in the test-and-treat subgroup achieved the desired health outcome that motivated first-line use of DTG/3TC and one (2%) did not; for the remaining three (5%), it was “too soon to tell” or the HCP was unsure if the desired health outcome had been achieved.
Treatment and Virologic Outcomes: High Baseline Viral Load Subgroups
For treatment-naive people with high baseline viral loads, patient preference was the most common HCP-reported reason for initiating DTG/3TC (identified for 5/16 [31%]; Fig. 1A). Additional reasons reported by HCPs were avoidance of long-term toxicities, convenience, and weight gain.
Among the 16 people with high baseline viral loads, all remained on DTG/3TC at data cutoff, for a median (IQR) treatment duration of 1.2 (0.8–1.8) years in the 100,000–250,000 copies/ml subgroup and 1.0 (0.7–1.1) years in the > 250,000 copies/ml subgroup (Table 3). Of all 16 individuals with baseline viral loads ≥ 100,000 copies/ml, 14 (88%) achieved virologic suppression and 13 (81%) remained virologically suppressed. More specifically, in the baseline viral load 100,000–250,000 copies/ml subgroup (median baseline CD4 + cell count, 312 cells/mm3), eight (89%) of nine achieved virologic suppression (HIV-1 RNA < 50 copies/ml), and all eight remained suppressed (one individual had missing data; Table 3, Fig. 2A). Among those with baseline viral loads > 250,000 copies/ml (median baseline CD4 + cell count, 114 cells/mm3), six (86%) of seven became virologically suppressed; five (71%) subsequently remained suppressed and one (14%) later experienced virologic rebound (two consecutive HIV-1 RNA measurements ≥ 200 copies/ml after suppression) but remained on DTG/3TC (one had missing data).
Median time to virologic suppression was 11.2 weeks for individuals with baseline viral loads 100,000–250,000 copies/ml and 20.6 weeks for those with baseline viral loads > 250,000 copies/ml (Table 3). The single individual who experienced virologic rebound had no resistance testing performed at DTG/3TC initiation. Healthcare providers reported that the desired health outcome (i.e., the outcome that motivated the selection of DTG/3TC as a first-line treatment option) was achieved in seven (78%) individuals with baseline viral loads 100,000–250,000 copies/ml and five (71%) with baseline viral loads > 250,000 copies/ml; for the remaining four individuals with baseline viral loads ≥ 100,000 copies/ml, it was “too soon to tell” or the HCP was unsure if the desired health outcome had been achieved.
Discussion
Outcomes were explored in subgroups of treatment-naive people with HIV-1 in TANDEM who initiated DTG/3TC in a test-and-treat setting or with high baseline viral loads (≥ 100,000 copies/ml), populations for which there is particular interest. The US Department of Health and Human Services guidelines recommend a test-and-treat approach, wherein ART is initiated soon after HIV diagnosis without availability of clinical information such as viral load, CD4 + cell count, and HIV-1 resistance data [12]. The potential benefits of rapidly initiating ART under a test-and-treat approach include increasing ART uptake and linkage to care, decreasing time to virologic suppression among newly diagnosed people with HIV, and reducing HIV transmission. However, the guidelines currently only recommend initiation of 3-drug regimens before baseline laboratory test results are available. Data on use of DTG/3TC in this setting were lacking, with one of the main concerns being the possibility of treatment failure and development of resistance when baseline viral load is high. Regardless, it is important to provide social, emotional, and educational support to individuals initiating treatment in a test-and-treat setting and closely monitor the effectiveness of ART regimens per guideline recommendations [12, 16,17,18]. Of 61 treatment-naive individuals in TANDEM who initiated DTG/3TC shortly after diagnosis in a test-and-treat setting, 57 (93%) achieved virologic suppression and 51 (84%) remained virologically suppressed after a median follow-up of 1.3 years; virologic suppression was achieved after a median of 9.7 weeks of DTG/3TC treatment. Sixty (98%) individuals in the test-and-treat subgroup remained on DTG/3TC at data cutoff. Avoidance of long-term toxicities, simplification/streamlining of treatment, and convenience were the most common HCP-reported reasons for DTG/3TC initiation. Results from the test-and-treat subgroup in TANDEM are in alignment with those from the phase 3b test-and-treat STAT clinical trial, in which 82% of participants in the ITT-E missing = failure analysis achieved virologic suppression at week 48. In a single-arm, multicenter, prospective trial in Spain, 76 (86%) of 88 treatment-naive people initiating DTG + 3TC within 1 week of initial consultation achieved HIV-1 RNA < 50 copies/ml at week 48 [19]. In a retrospective analysis of the REDOLA cohort in Spain, 111 (84%) of 132 treatment-naive people initiating DTG/3TC without availability of baseline resistance testing results had HIV-1 RNA < 50 copies/ml at week 96 [20]. One (< 1%) individual in the test-and-treat subgroup discontinued DTG/3TC in TANDEM; in STAT, one (< 1%) participant discontinued DTG/3TC because of an adverse event. Based on both clinical trial and real-world data, DTG/3TC is an effective and well-tolerated option for first-line ART in a test-and-treat setting.
In a separate analysis of treatment-naive individuals in TANDEM, 16 people with high baseline viral loads initiated DTG/3TC; 14 (88%) achieved virologic suppression (HIV-1 RNA < 50 copies/ml) and 13 (81%) remained virologically suppressed. Median time to achieve virologic suppression was 11.2 weeks for individuals in the baseline 100,000–250,000 copies/ml subgroup and 20.6 weeks in the baseline > 250,000 copies/ml subgroup. None of the 16 people with high baseline viral loads discontinued DTG/3TC after a median follow-up time of ≥ 1 year. Patient preference was the most common HCP-reported reason for initiating DTG/3TC in the high baseline viral load subgroup.
Results from TANDEM support 48-week results for DTG + 3TC in the phase 3 GEMINI-1/-2 clinical trials, in which 92% of treatment-naive participants with baseline viral loads > 100,000 copies/ml and 85% with baseline viral loads > 500,000 copies/ml achieved virologic suppression [11], and STAT, in which 82% of participants with baseline viral loads ≥ 100,000 copies/ml and 89% with baseline viral loads ≥ 500,000 copies/ml achieved virologic suppression at week 48 (ITT-E missing = failure analysis) [14]. No individuals with high baseline viral loads discontinued DTG/3TC in TANDEM; in the GEMINI trials, 2% (15/716) of participants discontinued at week 48 because of adverse events; the corresponding number in the STAT trial was < 1% (1/131) [8, 14]. In both clinical trial and real-world settings, DTG/3TC has been shown to be effective and well tolerated in people with high baseline viral loads.
Although real-world data are limited, there has been increasing evidence of the effectiveness of DTG/3TC in individuals with high baseline viral loads. An observational study of treatment-naive individuals in China who initiated DTG/3TC reported that 96% of 22 people with baseline viral loads ≥ 500,000 copies/ml achieved HIV-1 RNA < 50 copies/ml at week 48 [21]. In a single-arm, multicenter, prospective trial in Spain that included 17 treatment-naive individuals with baseline viral loads > 100,000 copies/ml, 14 (82%) achieved HIV-1 RNA < 50 copies/ml at week 48 [19]. Lastly, in a retrospective analysis of the REDOLA cohort in Spain, 39 (87%) of 45 people with baseline viral loads ≥ 100,000 copies/ml had HIV-1 RNA < 50 copies/ml at week 96 [20]. Together with the TANDEM results, these studies suggest that real-world effectiveness of DTG/3TC is consistent with efficacy results observed in clinical trial settings in those with high baseline viral loads.
These analyses have several limitations. TANDEM was a retrospective chart review; therefore, data may be missing or incomplete. Baseline hepatitis B virus status was not recorded. TANDEM captured treatment outcomes up to and including virologic rebound only; thus, there is no knowledge of outcomes post-rebound in the small number of people who experienced virologic rebound. Baseline viral loads were unavailable for the test-and-treat subgroup; had viral loads been captured, this population could have contributed to the size of the viral load subgroups. For the analyses by baseline viral load, only a small sample (n = 16) had documented viral loads ≥ 100,000 copies/ml. As no formal hypothesis testing was conducted, statistical comparisons between groups are not reported.
Conclusions
Results from subgroup analyses of the TANDEM study demonstrate that the 2-drug regimen DTG/3TC is effective, with few discontinuations, in real-world settings in treatment-naive people with HIV-1 under a test-and-treat approach and in those with high baseline viral loads, providing some of the first real-world data for DTG/3TC in such populations in the United States.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available due to privacy reasons. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
References
Juluca [prescribing information]. Durham, NC: ViiV Healthcare; 2022.
Dovato [prescribing information]. Durham, NC: ViiV Healthcare; 2023.
Biktarvy [prescribing information]. Foster City, CA: Gilead Sciences, Inc; 2022.
Katlama C, Ghosn J, Murphy RL. Individualized antiretroviral therapeutic approaches: less can be more. AIDS. 2017;31:1065–71.
Cento V, Perno CF. Two-drug regimens with dolutegravir plus rilpivirine or lamivudine in HIV-1 treatment-naive, virologically-suppressed patients: latest evidence from the literature on their efficacy and safety. J Glob Antimicrob Resist. 2020;20:228–37.
Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis. 2016;62:784–91.
Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs. 2017;7:113–4.
Cahn P, Sierra Madero J, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393:143–55.
Cahn P, Sierra Madero J, Arribas JR, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr. 2020;83:310–8.
Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy-naive adults with HIV-1 infection. AIDS. 2022;36:39–48.
Eron J, Hung C-C, Baril J-G, et al. Brief report: virologic response by baseline viral load with dolutegravir plus lamivudine vs dolutegravir plus tenofovir disoproxil fumarate/emtricitabine: pooled analysis. J Acquir Immune Defic Syndr. 2020;84:60–5.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed December 18, 2023.
Rolle C-P, Berhe M, Singh T, et al. Dolutegravir/lamivudine as a first-line regimen in a test-and-treat setting for newly diagnosed people living with HIV. AIDS. 2021;35:1957–65.
Rolle C-P, Berhe M, Singh T, et al. Sustained virologic suppression with dolutegravir/lamivudine in a test-and-treat setting through 48 weeks. Open Forum Infect Dis. 2023;10:ofad101.
Schneider S, Blick G, Burke C, et al. Two-drug regimens dolutegravir/lamivudine and dolutegravir/rilpivirine are effective with few discontinuations in US real-world settings: results from the TANDEM study. Infect Dis Ther. 2024. https://doi.org/10.1007/s40121-024-00961-y.
Florida Department of Health. HIV test and treat (T&T) and re-engage in care guidance. Florida Department of Health. https://www.floridahealth.gov/diseases-and-conditions/aids/clinical_resources/_documents/Test_and_Treat_Guidance.pdf. Accessed February 16, 2024.
New York State Department of Health. The new standard: treatment initiation at time of HIV diagnosis. https://www.health.ny.gov/diseases/aids/providers/treatment/docs/faqs.pdf. New York State Department of Health. Accessed February 16, 2024.
AETC National Coordinating Resource Center. Immediate ART initiation & restart: guide for clinicians. https://aidsetc.org/sites/default/files/media/document/2023-06/ncrc-rapid-art-full.pdf. AIDS Education & Training Center Program. Accessed February 16, 2024.
Hidalgo-Tenorio C, Pasquau J, Vinuesa D, et al. DOLAVI real-life study of dolutegravir plus lamivudine in naive HIV-1 patients (48 weeks). Viruses. 2022;14:524.
Pulido F, López Bernaldo de Quirós JC, Górgolas M, et al. 96 Weeks effectiveness and tolerability of DTG + 3TC in naive patients: the REDOLA study. Presented at HIV Drug Therapy Glasgow 2022; October 23–26, 2022; Glasgow, Scotland.
Zhao F, Rao M, Chen W, et al. Dolutegravir plus lamivudine dual-drug regimen in treatment-naive HIV-1-infected patients with high-level viral load: preliminary data from the real world. J Acquir Immune Defic Syndr. 2022;91(S1):S16–9.
Acknowledgements
The authors would like to thank the study investigators across each of the 24 US-based sites for their contributions toward the study in abstracting the data from medical charts of individuals who either initiated or switched to DTG/3TC. Data included in this manuscript have previously been presented in part at IDWeek 2022; October 19–23, 2022; Virtual and Washington, DC; Posters 1278 and 1279.
Medical Writing and Editorial Assistance
Editorial assistance was provided under the direction of the authors by Lisa Baker, PhD, CMPP, and Jennifer Rossi, MA, ELS, MedThink SciCom, and funded by ViiV Healthcare.
Funding
This study was funded by ViiV Healthcare (Durham, NC, USA). The journal’s Rapid Service Fee was funded by ViiV Healthcare.
Author information
Authors and Affiliations
Contributions
Cynthia Donovan, Gavin Harper, Deanna Merrill, Aimee A. Metzner, Katie Mycock, Hannah Wallis, Jimena Patarroyo, and Alan Oglesby contributed to the conception of the study. Paul Benson, Jennifer Kuretski, Cynthia Donovan, Gavin Harper, Deanna Merrill, Aimee A. Metzner, Katie Mycock, Hannah Wallis, Jimena Patarroyo, and Alan Oglesby contributed to the design of the study and the acquisition of data. Cynthia Donovan, Gavin Harper, Deanna Merrill, Aimee A. Metzner, Katie Mycock, Hannah Wallis, Andrew P. Brogan, Jimena Patarroyo, and Alan Oglesby contributed to the analysis and interpretation of data. Cynthia Donovan, Aimee A. Metzner, and Andrew P. Brogan contributed to drafting the manuscript. All authors contributed to revising the manuscript for important intellectual content and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of Interest
Paul Benson has received honoraria from ViiV Healthcare. Jennifer Kuretski has received honoraria from Gilead. Cynthia Donovan, Deanna Merrill, Aimee A. Metzner, Andrew P. Brogan, Jimena Patarroyo, and Alan Oglesby are employees of ViiV Healthcare and may own stock in GSK. Gavin Harper, Katie Mycock, and Hannah Wallis are employees of Adelphi Real World, which was contracted by ViiV Healthcare for this study.
Ethics Approval
Independent central institutional review board (IRB) ethical approval was granted by the Western IRB-Copernicus Group (WCG™ IRB, Princeton, NJ) on February 19, 2021 (reference number, 20210451). Subsequent ethics reviews were provided by WCG IRB for each site before initiation of data collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: Data included in this manuscript have previously been presented in part at IDWeek 2022; October 19–23, 2022; Virtual and Washington, DC; Posters 1278 and 1279.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Benson, P., Kuretski, J., Donovan, C. et al. Real-World Effectiveness of Dolutegravir/Lamivudine in People With HIV-1 in Test-and-Treat Settings or With High Baseline Viral Loads: TANDEM Study Subgroup Analyses. Infect Dis Ther 13, 875–889 (2024). https://doi.org/10.1007/s40121-024-00950-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00950-1