Abstract
Introduction
Long-term complications of chronic hepatitis B (CHB) viral infection, such as cirrhosis, hepatocellular carcinoma (HCC), and liver failure, cause a large disease burden. This study aimed to describe the epidemiology, clinical outcomes, and treatment patterns of CHB infection and co-infection with hepatitis D virus (HDV) in South Korea.
Methods
The retrospective, observational study used existing data from the Health Insurance Review and Assessment Service (HIRA) database. Confirmed cases of (CHB) and HBV/HDV co-infection were identified between 2013 and 2019. Hepatitis C virus co-infections and acute HBV infections were excluded. Incident cases diagnosed between 2015 and 2018 with no prior disease history up to 2 years were included. Patient characteristics, clinical outcomes, economic burden, and healthcare-resource utilization were described.
Results
The estimated 7-year prevalence of CHB and HBV/HDV co-infection were 0.9% and 0.0024%, respectively. The prevalence was higher among 45–54 years old (CHB: 1.6%, HBV/HDV: 0.0049%) and males (1.1%, 0.0035%). The 5-year cumulative incidences of compensated cirrhosis, decompensated cirrhosis, HCC, and liver transplantation were 13.3%, 7.1%, 8.4%, and 0.7%, respectively. Hyperlipidemia (40.6%), hypertension (23.5%), and peptic ulcer (23.7%) were the more prevalent comorbidities. Among CHB patients, 48.1% received ≥ 1 prescribed anti-HBV drug including interferon or nucleos(t)ide analogues and 64.4% had ≥ 1 hospitalization compared to 80.4% and 79.4% HBV/HDV patients. Estimated total healthcare costs for CHB and HBV/HDV were US$786 million and $62 million, respectively.
Conclusions
These findings provide insights to the epidemiology, clinical burden, treatment patterns, and healthcare costs of CHB and HBV/HDV co-infection in South Korea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic hepatitis B (CHB) is one of the causes predisposing liver damage and inflammation with an overall prevalence of 6.2% reported in 2019 in the Western Pacific adult population. |
This study assessed the epidemiology, disease burden, treatment patterns, and social costs of CHB and HBV/HDV co-infected patients in Korea using real-world evidence from the HIRA database. |
Most CHB and HBV/HDV co-infected patients were aged 45–54 years, were males, with hyperlipidemia and 48.1% received ≥ 1 prescribed anti-HBV drug. |
Early therapeutic intervention upon disease onset and novel targeted therapies are necessary to better prevent and decrease liver-related morbidity and mortality and to minimize the financial burden arising from CHB. |
Introduction
Viral hepatitis is an infection that causes liver inflammation and damage. Several different viruses cause hepatitis, including hepatitis A, B, C, D, and E. While hepatitis C virus (HCV) infection frequently leads to chronic liver disease, recent direct antiviral agents treatment can eradicate ≥ 98% of HCV-infected patients [1]. Nevertheless, chronic infection with hepatitis B virus (HBV) remains a critical risk worldwide, and it is still hard to achieve complete or functional cure. The World Health Organization (WHO) estimated 296 million individuals (3.9%) were living with chronic HBV infection in 2019 [2]. The rate of HBV infection was highest in WHO Western Pacific Regions (Taiwan, Japan, South Korea, and China), with 6.2% of the adult population infected. In 2016, the estimated South Korea prevalence was 3% with hepatitis B surface antigen (HBsAg)-positive rates between 0 and 3.5% depending on age groups [3]. HBV remains the predominant etiology of hepatocellular carcinoma (HCC), accounting for 62–75% of South Korea cases [4]. A systematic literature review of studies published until 2007 on chronic HBV infection had estimated the 5-year cumulative incidence of cirrhosis in hepatitis B e-antigen-positive and -negative hepatitis at 17% and 38%, respectively, in East Asia [5].
Disease progression and increased liver-related mortality are associated with non-liver comorbidities (e.g., alcohol abuse or obesity) and concurrent co-infections [e.g., hepatitis D virus (HDV)] [5,6,7]. In clinical aspects, HDV co-infection results either from simultaneous infection of HBV and HDV or from HDV following HBV. HBV/HDV co-infection is considered the most severe form of chronic viral hepatitis, causing rapid progression of cirrhosis and early HCC development [7, 8]. However, a lack of recent information regarding the epidemiology and public health burden of HBV/HDV co-infection in Korea persists [9,10,11].
Potent antiviral therapies have been directed towards decreased mortality of patients with chronic hepatitis B (CHB) and have increased overall survival by reducing the rate of progression to liver cirrhosis or developing HCC [12, 13]. Recently, the Korean Association for the Study of the Liver published clinical practice guidelines in chronic HBV management [14]. In South Korea, eight antiviral therapeutic options have been approved for chronic HBV treatment, including tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), besifovir dipivoxil maleate, and entecavir (ETV) [14]. Despite the use of potent oral nucleos(t)ide analogues (NA), NA single treatments hardly achieve complete cure of HBV infection, resulting in the disease burden remaining.
Significant economic burden also exists in Asian countries with increasing direct costs associated with the disease [15, 16]. In 2005, the total social costs of HBV infection, including direct and indirect costs, were estimated at near US$456 million; equivalent to ~ 4.0% of Korea’s national health expenditure [15], with a significant proportion accounted for by resources, including high costs incurred for medical, transportation, time, and productivity loss due to morbidity [15, 17].
This study aimed to provide up-to-date real-world data about CHB epidemiology, disease burden, treatment patterns, healthcare resource utilization (HCRU), and the associated social costs in South Korea using data from the Health Insurance Review and Assessment Service (HIRA). Additionally, this study also aimed to assess the impact of HBV/HDV co-infection on CHB disease burden and healthcare-related costs in South Korea.
Methods
Data Sources
This study used claims data from HIRA that covers 46 million patients per year, nearly 90.0% of South Korea’s total population. The database includes patient characteristics, inpatient or outpatient diagnoses, hospitalization details, healthcare services, and outpatient prescriptions [18]. The study was approved by the Institutional Review Board at the Korea National Institute for Bioethics Policy (P01-202106-21-008), and informed consent was waived due to the study nature of retrospective data analysis.
Study Design and Population
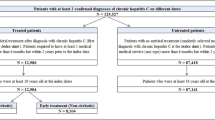
This retrospective observational study on patients with a diagnosis of CHB used existing data derived from the HIRA claims database. The study period was January 2012–October 2020. Patients were identified between the period of January 2013–December 2019. CHB patients were defined as: having ≥ 1 inpatient or ≥ 2 outpatient CHB diagnosis codes (KCD-10, B18.0 or B18.1) and with either ≥ 2 claims for the same disease-specific laboratory test [serologic testing—hepatitis B surface antigen (HBsAg), anti-hepatitis B core antibody (HBcAb), hepatitis B e antigen (HBeAg), HBV DNA, hepatitis B surface antibody (HBsAb), hepatitis B core-related antigen (HBcrAg), anti-hepatitis B e antibody (HBeAb), and HBV DNA mutation and genotype] occurring within a 1-year period or ≥ 1 reimbursement claims for interferon treatment (ATC codes starting with L03AB) or NAs (ATC codes starting with J05AF) of CHB. Patients with diagnosis codes of HCV or acute HBV infections were excluded.
To identify CHB patients with HDV co-infection (HBV/HDV patients), patients having ≥ 1 inpatient HDV or ≥ 2 outpatient HDV diagnosis codes and ≥ 1 claim for HDV antibody test occurring within the study period were also included in the study to minimize the overestimated population in the claims data (Fig. S1).
The enrollment period for patients within the prevalent cohort was January 2013–December 2019. The minimum follow-up period for the prevalent cohort was 10 months. Patients within the incident cohort, i.e., no history of disease (inpatient or outpatient diagnosis code of CHB) were evaluated longitudinally from the index date of which the enrollment period was January 2015–December 2018 and the minimum follow-up period was 22 months.
Baseline Variables
Demographics data included age and sex at baseline. Comorbidities of interest in the current study included hypertension, diabetes, hyperlipidemia, peptic ulcer, and chronic kidney disease, among others.
The Charlson comorbidity index (CCI) score, utilized with administrative databases based on the presence of 17 comorbidities and their severity was determined; the higher the score, the greater the disease burden [19]. In this study, all diseases found in the CCI score were considered for calculation except for human immunodeficiency virus data. Comorbidities during the 12-month preceding index date were defined by ≥ 1 inpatient or outpatient diagnosis codes.
Study Outcomes
Treatment Patterns
Prescriptions for CHB and HBV/HDV co-infection medications were identified during the follow-up period, [immune-based therapies (e.g., interferon or pegylated interferon alpha-2a) and NAs (e.g., lamivudine, adefovir, telbivudine, ETV, TAF, TDF, and clevudine)]. An algorithm was developed to define treatment lines based on several assumptions: (1) all drugs administered within 15 days of treatment initiation were considered part of the same regimen and line of therapy; (2) a new line of therapy was defined as either a new drug added following a 15-day initiation overlap or a 91-day gap between two different prescriptions (whichever was first) advancing the therapy line; the 91-day gap of prescription defined the discontinuation of previous drug(s); and (3) two consecutive lines containing the same drug(s) (e.g., regimen discontinued and restarted after a 91-day gap) were considered as one line. The maximum number of lines analyzed were three (Fig. S2).
Health Status
Patients were stratified based on the following health status: CHB, compensated cirrhosis (CC), decompensated cirrhosis (DC), and HCC with adapted definitions [6]. If > 1 diagnosis code existed with the same date, the patient was assigned to more than one health state.
Healthcare Resource Utilization
The economic burden was evaluated by assessing the HCRU and associated direct costs for the population, from service of care (inpatient and outpatient including all hospitalization, pharmacy, screening, physician visit cost) and by each health state depending on disease progression from CHB, CC, DC, HCC, LT until the end of follow-up.
Monetary values are presented in Korean Won (KRW) with value equivalent to USD through implementing the average official exchange rate in 2010–2020 as 1 KRW = USD 0.00089 [20].
Statistical Analysis
All study variables were examined using standard descriptive statistics. Categorical variables were shown as numbers and percentages, while continuous parameters were shown as the means [standard deviation (SD)] or medians [interquartile range (IQR)].
The prevalence of CHB in HIRA was determined as the total number of cases with an index date falling during this period divided by the average HIRA population size during this period. Age- and gender-specific prevalence were also estimated by average HIRA population size during this period. The prevalence was estimated where average population size by each age group and gender were used as denominators. All analyses were performed using SAS 9.4.
Results
Prevalence and Annual Incidence
A total of 444,203 CHB patients met the case definition of chronic HBV infection during 2013–2019. The overall 7-year prevalence was estimated at 0.9%. The prevalence of CHB increased with age, peaking at ages 45–54 years (1.6%), followed by 55–64 years (1.3%), and was higher in males (1.1%) than females (0.7%) (Table 1).
Among the prevalent cohort, 1175 were identified to have HDV co-infection, and the overall HBV/HDV co-infection prevalence was estimated at 0.0024% and 0.3% in the CHB-infected patient cohort. The aged-specific co-infection prevalence was highest among patients aged 45–54 years (0.0049%) and the sex-specific prevalence was higher in males (0.0035%) than females (0.0013%) (Table 1).
Demographic Characteristics and Comorbidity Burden
Table 2 described the patients’ characteristics and comorbidity burden between 2015 and 2018. The incident cohort comprised of 64,707 CHB patients, and 56.1% were males. The median age of incident CHB patients at index age was 48 years (IQR, 39–57) and 61.1% (n = 39,532) were aged ≥ 45 years. The majority of CHB patients were diagnosed at a clinic (n = 27,471, 42.5%), and 46.1% (n = 29,840) had mild liver disease at index date. The mean CCI score was 1.34 (SD: 1.86) and 57.0% have ≥ 1 comorbidity defining the CCI. Hyperlipidemia (40.6%), hypertension (23.5%), and peptic ulcer (23.7%) were the top 3 most prevalent comorbidities among incident patients. The majority of CHB patients (n = 61,477, 95.0%) were on ≥ 1 concomitant drug with 98.5% (n = 60,534) on non-steroidal anti-inflammatory drugs, 74.5% (n = 45,821) on proton pump inhibitors, and 25.7% on angiotensin receptor blockers (n = 15,790).
There were 194 HBV/HDV incident cases (0.3% among overall CHB population), and 65.5% (n = 127) were males. The median age of co-infected patients was 48 (IQR, 38–56), and 27.9% aged 45–54 years. About one-third of patients visited clinic (33.0%) or general hospital (37.2%). Among HBV/HDV patients, 93.3% (n = 181) were on ≥ 1 concomitant drug, with the top three prevalent drugs being non-steroidal anti-inflammatory drugs (n = 176, 97.2%), proton pump inhibitors (n = 154, 85.1%), and aspirin (n = 46, 25.4%).
Health States and Risk Factors
The median follow-up period of the incident cohort from 1st diagnosis was 58.8 months (IQR, 45.6–63.6). The 5-year cumulative incidence [95% confidence interval (CI)] of CHB patients developing CC, DC, HCC, and LT were estimated at 13.3% (13.0; 13.6), 7.1% (6.9, 7.3), 8.4% (8.2, 8.7), and 0.7% (0.6, 0.7), respectively. During the follow-up period, 12.5% of CHB patients developed CC, 6.7% developed DC, 7.9% developed HCC, and 0.6% underwent LT. The distribution of HBV/HDV patients’ health states of experiencing CC, DC, HCC, and LT were 35.5% (n = 60), 29.4% (n = 52), 15.6% (n = 27), and 17.4% (n = 32), respectively (Table 3). CHB patients being female, diagnosed with hypertension or hypercholesterolemia had a lower risk of developing CC, DC, HCC, and LT (all p < 0.0001). CCI was positively associated with DC and LT, while diabetes was positively associated with HCC (all p < 0.0001) (Table S1).
Treatment Patterns
Table 4 describes the treatment patterns of chronic HBV medications during the observation period of CHB and HBV/HDV patients. During the follow-up observation period, 48.1% (n = 31,155) of CHB patients and 80.4% (n = 156) of HBV/HDV patients received ≥ 1 prescribed CHB medication with NAs being the most predominant medication. The most used NAs by CHB patients were TDF (n = 20,070, 64.4%), ETV (n = 10,161, 32.6%), and TAF (n = 2620, 8.4%). The mean treatment duration (months) of patients on TDF, ETV, and TAF were 27.5, 24.1, and 15.6, respectively. Among HBV/HDV patients, TDF (n = 99, 63.5%), ETV (n = 67, 42.9%), and TAF (n = 13, 8.3%) were the most common NAs used; one HBV/HDV patient was on pegylated interferon alpha-2a. The mean treatment duration among the patients for TDF, ETV, and TAF were 28.5, 22.5, and 12.6 months, respectively. Most CHB patients had a medication possession ratio (MPR) > 90% for ETV (n = 8131/10,161, 80.0%), TAF (n = 2300/2,620, 87.8%), and telbivudine (n = 76/85, 89.4%). Among patients who received ≥ 1 medication, discontinuation was observed in 15,606 CHB patients (50.1%) and 83 HBV/HDV patients (53.2%). The proportions of medication switching by CHB patients on TDF, ETV, and TAF were 8.4% (n = 862/10,245), 10.0% (n = 549/5,509), and 19.7% (n = 109/553); HBV/HDV patients also switched medications while on TDF (n = 19/53), ETV (n = 21/36), and TAF (n = 2/4) (Table S2). Throughout the end of the observation period for CHB patients, 73.0% (n = 22,755/31,155) in first-line therapy received only one line of therapy, 6014 (19.3%) patients progressed to second-line therapy only, and 2,386 (3.7%) progressed to third-line (Fig. 2a). Among HBV/HDV patients, 102 (65.4%) received only first-line therapy (ETV, LAM, TDF, TAF, and immune-based therapies), 37 (23.7%) had only second-line therapy (ETV, TDF, TAF), and 17 (10.9%) progressed to third line (ETV and TDF) (Fig. S2b).
Resource Utilization and Costs
Table 5 describes HCRU and costs among CHB and HBV/HDV incident cohorts. Within the CHB incident cohort, 64.4% required ≥ 1 hospitalization and the median duration of hospitalization was 5 days (IQR 2–10). The highest number of hospitalizations (per patient-year) observed among patients with DC (1.93), LT (1.91), and HCC (1.28). The majority of patients with greater disease severity required ≥ 1 medication (DC: 83.5%; HCC: 84.1%; LT: 90.2%), or ≥ 1 serologic testing (99.8%; 99.9%; 100%), or ≥ 1 screening and monitoring test (all 100%), or ≥ 1 outpatient visit (99.7%; 99.9%; 99.5%).
Within the HBV/HDV incident cohort, 154 patients (79.4%) had ≥ 1 hospitalization and the median duration of hospital stay was 5 (IQR, 3–12). The number of hospitalizations per patient-year was 1.06, and 99.5% of patients had ≥ 1 outpatient visit, and all patients (100%) had ≥ 1 serologic test and screening and monitoring test.
The total healthcare costs for the CHB incident cohort were estimated to 882,972,409,170 KRW (approximately USD 786 million) during the follow-up period, with 57.2% due to inpatient costs (505,066,069,440 KRW [approximately USD 450 million)]. The total costs decreased with increasing severity, of which the total costs for CC health state (188,547,681.740 KRW (approximately USD 168 million) were the highest, while the total costs for LT health state was the lowest (69,184,900,590 KRW [approximately USD 62 million)]. The total healthcare costs for HBV/HDV incident cohort were 8,343,008,730 KRW (approximately USD 7.43 million) with inpatient costs estimated at 5,137,062,130 KRW (approximately USD 2.85 million).
Discussion
This extensive nationwide study in Korea investigated the real-world practice of CHB from multiple aspects using data from HIRA, and is also one of the first papers investigating a large dataset of HBV/HDV co-infection, assessing their clinical profile and socioeconomics of patients, treatment options, and helping to understand the resources required in healthcare to manage CHB in Korea.
The overall prevalence rate in our study was inconsistent with previous literature, where it was higher compared to the lower rates we observed [21]. The contrasting results between the studies could be due to the claim extraction from the national health insurance system as opposed to estimations on survey findings that project the overall population [22]. Albeit still an increased number of CHB patients in treatment phase, the decrease in prevalence could be indicative of potential success in measures to limit and control HBV infection in South Korea. Such measures include universal vaccination programs and other preventative strategies [23] that precluded both vertical and horizontal transmission, accounting for the decline in prevalence to those aged below 20 years old [24]. Additionally, the higher CHB and CHB/HBV co-infection prevalence rates observed in the individuals aged 45–50 years (born between 1970 and 1975) could potentially be attributed to the implementation of HBV control policies in South Korea that occurred only after the 1980s. Despite differences observed in the prevalence, the incidence rate was consistent with previous data collected from 2015 to 2020 [21]. This could potentially be attributed to the introduction of additional NA antivirals (lamivudine in 1999, adefovir in 2004, entecavir in 2007, tenofovir in 2012) and vaccination programs in 1984 that would require time for establishing optimal management and treatment strategies for CHB patients, and in order for the effects to reflect on the patient population [25].
The age of CHB patients could be explained by the rapidly aging population observed in Korea since 2000 [6, 26], as well as the HBV infection with potent NAs therapy rarely progressing to cirrhosis, leading to prolonged survival of patients. Hyperlipidemia was of the highest prevalence, followed by hypertension and peptic ulcer. CHB patients have shown higher proportions of metabolic disorders including hyperlipidemia, hypertension, and diabetes, as observed previously [27,28,29].
Consistent with previous epidemiological studies, there was a higher risk of cirrhosis, HCC [30], and liver failure in males in South Korea [31, 32]. Previous studies have reported that comorbidities and metabolic syndromes, such as hypertension, hypercholesterolemia, and diabetes, are associated with liver-related mortality [33,34,35]. Of note, hypertension and hypercholesterolemia were found to be negatively associated with the health states of chronic HBV. This could be attributed to the concomitant medications prescribed to the patients in this study; for instance, aspirin and statins use have been associated with lower risk of liver-associated mortality [35, 36].
The prevalence and incidence rate of HDV co-infection was 0.26% (1175/444,203) and 0.3% (n = 194/64,707), respectively, with the prevalence similar to previous reports (0.3%) [9]. Although the literature on incidence rate remains limited, it is noteworthy that HDV/HBV co-infection predisposes to advanced liver diseases, especially HCC development. HDV co-infection worsens pre-existing HBV-related liver injury. About 70–80% of co-infected patients develop chronic liver diseases [37] with up to three times risk of cirrhotic patients developing HCC [37, 38].
Approximately half of CHB patients received ≥ 1 prescribed medication in the incident cohort, lower than the WHO guidelines implicating an 80% increase in treatment rate by 2030 [39]. Prescription of CHB medication requires serological testing to be conducted. Additionally, patients in the immune-inactive phase (serum HBV DNA < 2000 IU/mL, normal alanine aminotransferase levels, and no cirrhosis) are excluded from the regimen. At present, antiviral treatment in South Korea is determined by severity of liver disease, degree of HBV replication in hepatitis B e antigen-positive or -negative individuals, and the presence of liver fibrosis [14].
First-line therapies comprising of ETV and TDF were the most prescribed drugs in first-line treatment for both CHB and HBV/HDV patients. Extensive clinical data on ETV and TDF implicate their long-term efficacy and safety [14]. Moreover, HDV co-infection recommends inclusion of tenofovir in highly active antiretroviral therapy, and that patients be treated with pegylated interferon alpha-2a for at least a year. Initiation of NAs for CHB is recommended to prevent progression of fibrosis if either indications for treatment are met or liver cirrhosis occurs [14]. Management is based on an algorithm (Table S2) that determines initiation, switching, or discontinuation of treatment [14]. Treatment duration of over 2 years reportedly decreases the risk of HBV-related chronic liver disease progression in adult patients [40]. Of note, the average treatment duration in our study was approximately 1.5 years. While ≥ 70% of patients adhered to the treatment, the duration of adherence was not reported. Factors that could affect treatment adherence rates, e.g., socioeconomic disparity/status, health consciousness, family support, and concerns of long-term treatment safety [14, 41,42,43], were not explored in this study. Furthermore, the treatment pattern identified in this study was based on the incidence cohort of CHB and HBV/HDV co-infected patients diagnosed between 2013 and 2019 and met the inclusion and exclusion criteria. While the findings could be representative of those diagnosed between 2013 and 2019, future investigation would be warranted to understand the representation of our findings.
The total societal costs of CHB were estimated to KRW 833 billion (approximately USD 786 million with the majority attributed towards inpatient costs. Several studies have reported similar findings [16, 44, 45], where hospitalization costs were the economic driver in most of health states due to disease severity. The impact of co-infection further increases this burden based on the disease severity that requires a longer treatment and hospitalization length [46, 47].
The strengths of this study determined the prevalence of CHB in South Korea using a national claims database. The additional findings of HBV/HDV co-infection uses a large dataset in South Korea and real-world evidence from the HIRA database, which, unlike other claims databases restricted to hospital data, allows longitudinal tracking of patients across different medical institutions. Additionally, the large sample size and long follow-up period strengthen the findings from multiple aspects related to the disease. As this study provides an overview of the disease landscape from a real-world perspective, future studies could potentially explore the association between the effort of preventive programs, or the introduction of NA since 1999 and the prevalence and incidence rate of CHB and CHB/HDV co-infection among the South Korea population.
There are limitations to this study. It used existing data from the HIRA database of patients who met the inclusion criteria and was based on data extracted between the study period of 2013–2019. Additionally, while the HIRA database captured the number (and types) of claims for CHB-specific laboratory tests, the diagnostic data or laboratory test results were unavailable [48], thus limiting the ability to validate the CHB diagnosis or the ability to investigate the impact of HBV mutations on CHB disease burden.
In terms of identifying HDV infections within the CHB population, the claim data did not permit distinguishing between acute or chronic HDV infection. Moreover, data pertaining to individuals who did not receive surveillance or treatment for CHB and/or HBV/HDV co-infection were unavailable in this study. Future studies may be warranted to investigate the impact of acute versus chronic HDV co-infection on the disease burden associated with CHB. In addition, incident cases were based on the 2-year period before the index (disease history determination), and is considered short for history detection for a chronic disease, especially in the initial stages. The epidemiology of HDV infection may be underestimated, as the prevalence and incidence rates of HDV-diagnosed patients were derived from those who had CHB in the claims database instead of through HDV actively screened among CHB patients. Therefore, it may not be representative of South Korea’s national population which was obtained from the HIRA. It is noteworthy that first-line treatment definition was assumption-based. Additionally, no verification of the patient was already on the treatment before the study and the reasons pertaining to discontinuation or switch remain unknown.
Conclusion
This study is one of the first using real-world evidence based on the data obtained from HIRA, addressing the epidemiology, disease burden, treatment patterns, and social costs of CHB and HBV/HDV co-infected patients in Korea. Albeit a decrease was observed in prevalence across the years, the disease remains a challenge in healthcare due to its high economic burden arising from increased morbidity, and issues as the extent of the disease progresses towards liver-related diseases. Early intervention at disease onset and novel targeted therapies are warranted to help better prevent and decrease liver-related morbidity and mortality and to minimize the financial burden arising from it.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
Manns MP, Maasoumy B. Breakthroughs in hepatitis C research: from discovery to cure. Nat Rev Gastroenterol Hepatol. 2022;19(8):533–50.
World Health Organization. Hepatitis B [Internet]. Hepatitis B. 2022. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed 11 Oct 2022.
Kim DY. History and future of hepatitis B virus control in South Korea. Clin Mol Hepatol. 2021;27(4):620–2.
Yim SY, Kim JH. The epidemiology of hepatitis B virus infection in Korea. Korean J Intern Med. 2019;34(5):945–53.
Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–52.
Oh H, Jun DW, Lee IH, Ahn HJ, Kim BO, Jung S, et al. Increasing comorbidities in a South Korea insured population-based cohort of patients with chronic hepatitis B. Aliment Pharmacol Ther. 2020;52(2):371–81.
Shukla NB, Poles MA. Hepatitis B virus infection: co-infection with hepatitis C virus, hepatitis D virus, and human immunodeficiency virus. Clin Liver Dis. 2004;8(2):445–60.
Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7(6):402–9.
Kim HS, Kim SJ, Park HW, Shin WG, Kim KH, Lee JH, et al. Prevalence and clinical significance of hepatitis D virus co-infection in patients with chronic hepatitis B in Korea. J Med Virol. 2011;83(7):1172–7.
Sagnelli C, Sagnelli E, Russo A, Pisaturo M, Occhiello L, Coppola N. HBV/HDV co-infection: epidemiological and clinical changes, recent knowledge and future challenges. Life (Basel). 2021;11(2):169.
Hayashi T, Takeshita Y, Hutin YJF, Harmanci H, Easterbrook P, Hess S, et al. The global hepatitis delta virus (HDV) epidemic: what gaps to address in order to mount a public health response? Arch Public Health. 2021;79(1):180.
Kim WR, Loomba R, Berg T, Aguilar Schall RE, Yee LJ, Dinh PV, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121(20):3631–8.
Yuen MF, Seto WK, Chow DHF, Tsui K, Wong DKH, Ngai VWS, et al. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12(8):1295–304.
Korean Association for the Study of the Liver. KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019;25(2):93–159.
Shon C, Choi HY, Shim JJ, Park SY, Lee KS, Yoon SJ, et al. The economic burden of hepatitis A, B, and C in South Korea. Jpn J Infect Dis. 2016;69(1):18–27.
Hsieh CR, Kuo CW. Cost of chronic hepatitis B virus infection in Taiwan. J Clin Gastroenterol. 2004;38(10 Suppl 3):S148-152.
Yang BM, Kim DJ, Byun KS, Kim HS, Park JW, Shin S. The societal burden of HBV-related disease: South Korea. Dig Dis Sci. 2010;55(3):784–93.
Yuen MF, Yuan HJ, Wong DKH, Yuen JCH, Wong WM, Chan AOO, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54(11):1610–4.
Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–40.
South Korean Won to US Dollar Spot Exchange Rates for 2010 [Internet]. 2022. https://www.exchangerates.org.uk/KRW-USD-spot-exchange-rates-history-2010.html. Accessed 7 Oct 2022.
Le LV, Blach S, Rewari B, Chan P, Fuqiang C, Ishikawa N, et al. Progress towards achieving viral hepatitis B and C elimination in the Asia and Pacific region: Results from modelling and global reporting. Liver Int. 2022;42(9):1930–4.
Son HE, Jung SJ, Shin A. Health Screening among HBV Carriers in the Korean National Health and Nutrition Examination Survey V (KNHANES V). Asian Pac J Cancer Prev. 2015;16(9):3653–7.
Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010;53(1):20–8.
Hann HWL, Hann RS, Maddrey WC. Hepatitis B virus infection in 6,130 unvaccinated Korean-Americans surveyed between 1988 and 1990. Am J Gastroenterol. 2007;102(4):767–72.
Choi MS, Sinn DH, Kim SA, Lee YS, Choi W, Paik SW. The clinical and laboratory characteristics of patients with chronic hepatitis B using current or past antiviral therapy in Korea: a multi-center, nation-wide, cross-sectional epidemiologic study. Gut Liver. 2012;6(2):241–8.
Cho Y, Bo HK, Joong-Won P. The emerging age-pattern changes of patients with hepatocellular carcinoma in Korea. Clin Mol Hepatol. 2022;29(1):99–101.
Liu A, Le A, Zhang J, Wong C, Wong C, Henry L, et al. Increasing co-morbidities in chronic hepatitis B patients: experience in primary care and referral practices during 2000–2015. Clin Transl Gastroenterol. 2018;9(3):141.
Khalili M, Lombardero M, Chung RT, Terrault NA, Ghany MG, Kim WR, et al. Diabetes and prediabetes in patients with hepatitis B residing in North America. Hepatology. 2015;62(5):1364–74.
Liu S, Zhang H, Gu C, Yin J, He Y, Xie J, et al. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst. 2009;101(15):1066–82.
Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28(4):583–705.
Shin BM, Yoo HM, Lee AS, Park SK. Seroprevalence of hepatitis B virus among health care workers in Korea. J Korean Med Sci. 2006;21(1):58–62.
Kim GA, Lee HC, Kim MJ, Ha Y, Park EJ, An J, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J Hepatol. 2015;62(5):1092–9.
Song BG, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Changes in the prevalence of hepatitis B and metabolic abnormalities among young men in Korea. Korean J Intern Med. 2022;37(5):1082–7.
Lee YB, Moon H, Lee JH, Cho EJ, Yu SJ, Kim YJ, et al. Association of metabolic risk factors with risks of cancer and all-cause mortality in patients with chronic hepatitis B. Hepatology. 2021;73(6):2266–77.
Jang H, Lee YB, Moon H, Chung JW, Nam JY, Cho EJ, et al. Aspirin use and risk of hepatocellular carcinoma in patients with chronic hepatitis B with or without cirrhosis. Hepatology. 2022;76(2):492–501.
Vargas JI, Arrese M, Shah VH, Arab JP. Use of statins in patients with chronic liver disease and cirrhosis: current views and prospects. Curr Gastroenterol Rep. 2017;19(9):43.
Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The european concerted action on viral hepatitis (Eurohep). Gut. 2000;46(3):420–6.
Stroffolini T, Ciancio A, Furlan C, Vinci M, Niro GA, Russello M, et al. Chronic hepatitis B virus infection in Italy during the twenty-first century: an updated survey in 2019. Eur J Clin Microbiol Infect Dis. 2021;40(3):607–14.
Toy M, Hutton D, Jia J, So S. Costs and health impact of delayed implementation of a national hepatitis B treatment program in China. J Glob Health. 2022;8(12):04043.
Zhang QQ, An X, Liu YH, Li SY, Zhong Q, Wang J, et al. Long-term nucleos(t)ide analogues therapy for adults with chronic hepatitis B reduces the risk of long-term complications: a meta-analysis. Virol J. 2011;8:72.
Chotiyaputta W, Hongthanakorn C, Oberhelman K, Fontana RJ, Licari T, Lok ASF. Adherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughs. J Viral Hepat. 2012;19(3):205–12.
Giang L, Selinger CP, Lee AU. Evaluation of adherence to oral antiviral hepatitis B treatment using structured questionnaires. World J Hepatol. 2012;4(2):43–9.
Xu K, Liu LM, Farazi PA, Wang H, Rochling FA, Watanabe-Galloway S, et al. Adherence and perceived barriers to oral antiviral therapy for chronic hepatitis B. Glob Health Action. 2018;11(1):1433987.
Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, et al. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021;27(3):363–401.
Yang S, Chen G, Li Y, Li G, Liang Y, Zhou F, et al. The trend of direct medical costs and associated factors in patients with chronic hepatitis B in Guangzhou, China: an eight-year retrospective cohort study. BMC Med Inform Decis Mak. 2021;21(Suppl 2):71.
Rizzetto M, Hamid S, Negro F. The changing context of hepatitis D. J Hepatol. 2021;74(5):1200–11.
Wasuwanich P, Striley CW, Kamili S, Teshale EH, Seaberg EC, Karnsakul W. Hepatitis D-associated hospitalizations in the United States: 2010–2018. J Viral Hepat. 2022;29(3):218–26.
Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of korea health insurance review and assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–28.
Acknowledgements
Medical Writing Assistance
The authors thank Dr. Fatima Megala Nathan Arokianathan from Cerner Enviza for providing medical writing and editorial support. This support was funded by Janssen Asia Pacific.
Funding
This study, including the journal’s Rapid Service fee, was funded by Janssen Asia Pacific. Cerner Enviza received funding from Janssen Asia Pacific during the conduct of the study and the development of the manuscript and has no other funding, financial relationships, or conflicts of interest to disclose.
Author information
Authors and Affiliations
Contributions
Y Cho, SB Park, SY Park and WJ Choi conceived the idea. Y Cho, SB Park, SY Park, WJ Choi, BO Kim, and H Han contributed to the design of the study, analysis and interpretation of data and drafting the article. BO Kim and H Han contributed to the acquisition of data and revising the article. Y Cho, SB Park, SY Park, WJ Choi, BO Kim, and H Han participated in the analysis and interpretation of data and revision the article. All authors gave their final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of Interest
Yuri Cho has nothing to disclose. BoOk Kim and Helin Han are employees of Cerner Enviza and have nothing to disclose. SeongBeom Park, SeonYoung Park and WonJung Choi are employees of Janssen, South Korea. There are no conflicts to disclose.
Ethical Approval
This study was approved by the Institutional Review Board at the Korea National Institute for Bioethics Policy (P01-202106-21-008) and informed consent was waived due to the study nature of retrospective data analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cho, Y., Park, S., Park, S. et al. Real-World Epidemiology, Treatment Patterns, and Disease Burden of Chronic Hepatitis B and HDV Co-Infection in South Korea. Infect Dis Ther 12, 2387–2403 (2023). https://doi.org/10.1007/s40121-023-00860-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00860-8