Abstract
Carbapenem-resistant Enterobacterales (CRE) remain a significant public health threat, and, despite recent approvals, new antibiotics are needed. Severe infections caused by CRE, such as nosocomial pneumonia and bloodstream infections, are associated with a relatively high risk of morbidity and mortality. The recent approval of ceftazidime–avibactam, imipenem–relebactam, meropenem–vaborbactam, plazomicin, eravacycline and cefiderocol has broadened the armamentarium for the treatment of patients with CRE infections. Cefiderocol is a siderophore cephalosporin with overall potent in vitro activity against CRE. It is taken up via iron transport channels through active transport, with some entry into bacteria through traditional porin channels. Cefiderocol is relatively stable against hydrolysis by most serine- and metallo-beta-lactamases, including KPC, NDM, VIM, IMP and OXA carbapenemases—the most frequent carbapenemases detected in CRE. The efficacy and safety of cefiderocol has been demonstrated in three randomised, prospective, parallel group or controlled clinical studies in patients at risk of being infected by multidrug-resistant or carbapenem-resistant Gram-negative bacteria. This paper reviews the in vitro activity, emergence of resistance, preclinical effectiveness, and clinical experience for cefiderocol, and its role in the management of patients with CRE infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Infections caused by carbapenem-resistant Enterobacterales (CRE) remain a challenging threat to public health, despite recent achievements in antibiotic development. |
A range of resistance mechanisms exist in CRE, which makes antibiotic development challenging, as new antibiotics may overcome only some mechanisms and emerging resistance could also hinder their long-term use. |
Cefiderocol is a siderophore cephalosporin with a unique mode of entry into Gram-negative bacteria, which has shown potent in vitro activity against CRE with any of the major carbapenem-resistance mechanisms from global collections. |
Cefiderocol was efficacious in the treatment of Gram-negative bacterial infections caused by CRE strains producing metallo-beta-lactamases, Klebsiella pneumoniae carbapenemase and OXA-48 enzymes. |
Cefiderocol has been approved in the USA for the treatment of adult patients with ventilator-associated bacterial pneumonia, hospital-acquired bacterial pneumonia, and complicated urinary tract infections, and in Europe for the treatment of adult patients with infections caused by susceptible Gram-negative bacteria with limited treatment options. |
Introduction
Burden of Carbapenem-Resistant Enterobacterales
Carbapenem-resistant (CR) Enterobacterales (CRE), particularly CR Klebsiella pneumoniae and CR Escherichia coli, constitute a major global health threat [1,2,3]. According to the World Health Organization (WHO), between 2016 and 2020 there was a significantly increasing trend in the carbapenem resistance rate among E. coli and K. pneumoniae [2], with some countries reporting prevalence as high as ≥ 50% for CR K. pneumoniae [2]. CRE infections may emerge among patients hospitalised in acute care hospitals, in long-term care facilities, and in patients in community settings [4,5,6,7]. Data from prospective studies in the US (n = 1040) and China (n = 128) report that, among hospitalised patients, CRE most commonly cause urinary tract infections (UTI) and lower respiratory tract infections, followed by bloodstream infections (BSI), wound infections and, less frequently, intra-abdominal infections (IAI) [7, 8]. Colonisation by CRE of mucosal surfaces is one of the risk factors for acquiring subsequent infections; thus, active screening may play a role not only in infection control but also for appropriate patient management and antibiotic stewardship [7,8,9].
Risk factors for CRE colonisation and/or infection are similar to those identified for all CR Gram-negative infections [10], being associated with the host (e.g., older age), hospitalisation (e.g., number of previous hospitalisations, emergency department stay > 2 days prior to intensive care unit admission), treatment (e.g., previous exposure to antibiotics) and procedures (e.g., invasive procedures/indwelling devices), recent surgery, immunocompromised status and organ transplantation [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25], chronic skin ulcers [13], along with mechanical ventilation and patient movement between hospital departments [26].
Evidence suggests that delay in appropriate antibiotic treatment of CRE infections increases the risk of morbidity and mortality [27]. The prospective, multicentre, CRACKLE-2 cohort study found that the mortality rate among 449 US hospitalised patients with CRE infections was 24% [7], although earlier studies have shown mortality rates > 40% [28, 29]. More vulnerable patient populations, such as those undergoing solid organ transplantation, may be at a higher risk of CRE-related recurrent infections and hospitalisations, increasing the risk of morbidity and mortality [30].
The main mechanism of carbapenem resistance in Enterobacterales is due to the production of various carbapenem-hydrolysing enzymes: also called carbapenemases. The proportion of carbapenemase-producing (CP) strains among CRE varies between regions (i.e., 76.2–90%) and a smaller proportion are non-CP CRE [7, 31, 32]. However, in certain geographical areas, non-CP CRE may yet be more prevalent (e.g., Texas) [33], while an increasing spread of carbapenemases was found in Europe between 2013 and 2017 [34]. Mortality findings for infections due to CP-CRE versus non-CP CRE are equivocal. Some investigators have reported a higher likelihood of mortality for non-CP CRE compared with CP CRE infections [26, 32], whereas others have reported a higher mortality rate with CP CRE versus non-CP CRE bacteraemia [35], and still others have found no difference in mortality rates [7].
Mechanisms of Carbapenem Resistance
CRE may harbour a range of mechanisms that render these pathogens resistant to carbapenems, including: (1) production of serine-carbapenemases or metallo-carbapenemases; and (2) expression of extended-spectrum beta-lactamases (ESBLs) and/or AmpC, which weakly hydrolyse carbapenems, in association with impaired drug uptake due to loss or modification of porin channels and/or upregulation of efflux pumps [36,37,38,39]. This variety of CRE resistance mechanisms poses a challenge to the selection of even novel antibiotics, most of which are not active against all mechanisms.
Carbapenemase production is the most widespread mechanism of carbapenem resistance owing to the high transmission rates via mobile elements (e.g., plasmids, transposons) and their frequent association with high-risk clones, which are particularly efficient in spreading. Carbapenemases may belong to distinct molecular classes of beta-lactamases [40,41,42,43]: Class A and Class D enzymes, which have a serine in their catalytic domain and exhibit variable hydrolytic activities towards carbapenems, and Class B enzymes [the metallo-beta-lactamases (MBLs)], which readily hydrolyse these antibiotics [36, 41]. The most notable plasmid-encoded Class A carbapenemase, Klebsiella pneumoniae carbapenemase (KPC), emerged over 25 years ago [44]. The Class D oxacillinase OXA-48 and its variants (e.g., OXA-162, -181, -204, -232, -244, and others) have been associated with CRE in the presence of permeability defects. Because Enterobacterales isolates can be reported as carbapenem susceptible when expressing OXA-48, their prevalence (and their spread) may be underestimated [45]. The most frequent MBLs include New Delhi (NDM), Verona integron-encoded (VIM), imipenemase MBL (IMP), and variants thereof [46, 47]. Class C serine-beta-lactamases (AmpC-type) primarily hydrolyse cephalosporins and only very weakly carbapenems; however, isolates expressing these plasmid-encoded enzymes or overproducing chromosomal AmpC in the presence of porin channel mutations or upregulated efflux pumps may become CR [41, 48]. Notable major porin channels are OmpC and OmpF in E. coli, and OmpK35 and OmpK36 in K. pneumoniae [49].
Methods
Literature Search Strategy
A literature search for this narrative review was conducted in PubMed for relevant published papers and in conference abstracts with the search terms “cefiderocol”, “carbapenem-resistant”, “CRE”, “Enterobacterales”, “Enterobacteriaceae”, “Klebsiella pneumoniae” and “Escherichia coli”. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Current Treatment Options for CRE
Historically, treatment options for infections caused by CRE included polymyxins, tigecycline, carbapenems, aminoglycosides and fosfomycin, as monotherapy or more frequently in combination therapy. It is concerning that resistance to colistin is spreading, and that approximately 20% of CRE isolates are resistant to colistin, according to surveillance studies involving isolates from the USA, Europe (i.e., 21.3%) and Japan (i.e., 20.4%) [50, 51]. Among the newer agents, ceftazidime–avibactam, meropenem–vaborbactam, imipenem–relebactam, plazomicin [for complicated UTI (cUTI) only in the USA], eravacycline [for complicated IAI (cIAI) only] and cefiderocol are approved for the treatment of patients with CRE infections when isolates are susceptible to these agents [52,53,54,55,56,57,58,59,60,61]. The effectiveness of these treatment options is related to specific CR mechanisms [37, 62,63,64].
Randomised, double-blind, non-inferiority, controlled studies enrolling patients with cUTI, cIAI, or nosocomial pneumonia (NP), and secondary bacteraemia have demonstrated the efficacy and safety of these antibiotics in certain patient populations, although these studies did not specifically target CRE infections [65,66,67,68,69,70,71,72]. On the other hand, data on the efficacy of these antibiotics in CRE infections were provided by smaller prospective, open-label or retrospective observational studies, or by small pathogen-focused, prospective, descriptive studies, which specifically targeted CR infections. The efficacies of meropenem–vaborbactam in TANGO II [73], plazomicin in CARE [74] and imipenem–relebactam in RESTORE-IMI 1 [75] were demonstrated and compared with either best available therapy (BAT) or colistin-based therapy, although the RESTORE-IMI 1 study only included six patients with CRE infections, limiting the evaluation of imipenem–relebactam efficacy against these pathogens [75]. These studies enrolled a limited number of patients due to the low prevalence of CRE globally and to the challenges related to enrolling such a medically complex patient population [2, 76]. Considering all clinical evidence [56, 73, 75, 77,78,79,80,81,82,83] and current in vitro activity [49, 50, 76, 84,85,86,87,88], the recently published European Society of Clinical Microbiology and Infectious Diseases (ESCMID) treatment guidelines and the Infectious Diseases Society of America (IDSA) guidance provide recommendations for use of these antibiotics in various infections complicated by each of the CR mechanisms [89, 90]. Additionally, country-specific recommendations in Europe may be considered according to local epidemiology [63, 64]. Further clinical evidence on the latest antibiotics is needed to strengthen the recommendations.
Cefiderocol
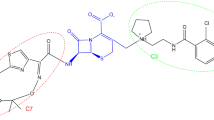
Cefiderocol is the first approved siderophore cephalosporin. It targets penicillin-binding proteins (PBPs), mainly PBP-3 [91]. The structure inherently enhances the stability of cefiderocol against hydrolysis by various beta-lactamases, including MBLs and other carbapenemases, thus enabling potential in vitro activity even in the absence of a beta-lactamase inhibitor [91]. The structure also allows iron chelation via the linked chlorocatechol group on the C-3 side chain, which consequently promotes the uptake of cefiderocol into the periplasmic space via active siderophore transport systems in Gram-negative bacteria [91]. The siderophore moiety of cefiderocol mimics natural siderophore molecules released by bacteria to facilitate iron uptake, which is essential for bacterial growth. Thus, cefiderocol predominantly enters the cell via active transport through iron transport systems, and a relatively small amount via traditional porins [91, 92]. Because cell entry is mediated by active transport during iron uptake by the bacteria, either porin channel loss or modification, or efflux pump upregulation has very limited impact on its in vitro activity [91, 92].
In Vitro Activity of Cefiderocol: Susceptibility Testing and Breakpoints, and Global Epidemiology
The reference method for in vitro susceptibility testing of cefiderocol is broth microdilution in iron-depleted cation-adjusted Mueller–Hinton medium [93, 94]. According to current European Committee for Antimicrobial Susceptibility Testing (EUCAST), clinical breakpoints for cefiderocol against Enterobacterales, susceptibility is a minimum inhibitory concentration (MIC) ≤ 2 µg/mL (or a ≥ 22-mm zone diameter for a 30-µg disk) and resistance is an MIC > 2 µg/mL (or a < 22-mm zone diameter for a 30-µg disk) [93]. The Clinical and Laboratory Standards Institute (CLSI) and Food and Drug Administration define susceptibility as ≤ 4 µg/mL (or a ≥ 16-mm zone diameter for a 30-µg disk), intermediate as 8 µg/mL (a 9- to 15-mm zone diameter for a 30-µg disk) and resistance as ≥ 16 µg/mL (or a ≤ 8-mm zone diameter for a 30-µg disk) [94]. Various commercial systems for cefiderocol susceptibility testing have also been developed, but they have been affected by major accuracy issues [95], resulting in a recent warning by EUCAST [96].
Cefiderocol is active against a broad range of aerobic Gram-negative bacteria, including Enterobacterales and non-fermenters, but lacks activity against Gram-positive and anaerobic bacteria. In an in vitro study, cefiderocol MIC values were found to be between ≤ 0.031 and 8 µg/mL for E. coli and K. pneumoniae clinical isolates that expressed ESBLs (MIC range 0.125–2 µg/mL), KPC (MIC range 2–8 µg/mL), NDM (MIC range 2–4 µg/mL), IMP (MIC range 0.125–1 µg/mL), OXA-48 (MIC ≤ 0.031 µg/mL) and acquired AmpC enzymes (MIC range 0.063–1 µg/mL) (Table 1) [92]. In the same study, deletion in iron transport proteins in E. coli strains led to an increase in cefiderocol MIC values; however, these isolates remained susceptible to cefiderocol based on current EUCAST and CLSI susceptibility breakpoints [92]. Similar findings were observed with mutations in porin channel ompK35/36 genes in K. pneumoniae, where two- to four-fold increases in cefiderocol MIC values were observed and the highest MIC value was 0.125 µg/mL [92].
The in vitro activity of cefiderocol was investigated in the multinational SIDERO-WT and SIDERO-CR surveillance programmes in clinical isolates collected between 2014 and 2020 (Table 1) [76, 97,98,99,100,101,102,103]. These surveillance programmes investigated the susceptibility of Gram-negative bacteria to cefiderocol and comparator antibiotics, including carbapenem-susceptible Enterobacterales, CRE and Enterobacterales isolates that were non-susceptible to ceftazidime–avibactam or ceftolozane–tazobactam (Table 1) [97,98,99,100,101,102,103]. Among meropenem-non-susceptible Enterobacterales, cefiderocol demonstrated a MIC90 value of 4 µg/mL in 2014–2015 [97], with regional variance (North America 1 µg/mL; Europe 4 µg/mL) [97]. The annual cefiderocol susceptibility rate among all Enterobacterales in the SIDERO programme according to CLSI criteria ranged between 99.6 and 100% in North America, and between 99.3 and 99.9% in Europe, with very small variations [76]. Among 1021 meropenem-non-susceptible Enterobacterales collected over 5 years, 96.7% (CLSI) and 79.9% (EUCAST) were susceptible to cefiderocol (i.e., the difference is due to EUCAST breakpoints categorising isolates with cefiderocol MICs of 4 μg/mL as resistant [~ 17%)] [76]. Additionally, 91.6% of isolates non-susceptible to ceftazidime–avibactam and 97.7% of isolates non-susceptible to ceftolozane–tazobactam were susceptible to cefiderocol based on CLSI breakpoints [76]. Among difficult-to-treat resistant Enterobacterales, 98.3% were susceptible to cefiderocol based on CLSI breakpoints (Table 1) [101].
Against 120 MBL-producing CRE isolates (including NDM and VIM) collected between 2014 and 2017 in the SIDERO-WT programme, the cefiderocol MIC90 was 4 µg/mL [102]. Against 45 NDM-producing CREs, MIC90 was 8 µg/mL (84% of isolates were inhibited at 4 µg/mL) and against 75 VIM-producing CREs, MIC90 was 4 µg/mL (100% of isolates were inhibited at 4 µg/mL) (Table 1) [102]. Additional data for 151 CRE isolates collected between 2014 and 2015 showed that the cefiderocol MIC90 values were 2 µg/mL for KPC-producing CRE, 2 µg/mL for carbapenemase-negative CRE, 4 µg/mL for VIM-producing CRE, 4 µg/mL for OXA-48-producing CRE and 8 µg/mL for NDM-producing CRE (Table 1) [100]. These isolates also had a high rate of colistin resistance (i.e., 30.5%) [100]. A European subset of the carbapenemase-producing (i.e., KPC, VIM, OXA-48 and NDM-1) strains collected in SIDERO-CR between 2014 and 2016 showed that, among 107 ceftazidime–avibactam-resistant Enterobacterales isolates, cefiderocol MIC values were ≤ 2 µg/mL (EUCAST breakpoint) for 66.4% of the isolates, approximately 30% had a MIC value of 4 µg/mL and only a few isolates were detected with MICs of 8 and 32 µg/mL [103]. The overall MIC90 value was 4 µg/mL for each carbapenemase type, suggesting a high cefiderocol susceptibility rate among CRE, including ceftazidime–avibactam-resistant strains (Table 1) [103].
Similarly, a very high susceptibility rate to cefiderocol was found in the SENTRY surveillance programme in CRE isolates collected in 2020 (Table 1) [50]. Overall, for Enterobacterales, cefiderocol demonstrated MIC50/MIC90 values of 0.06/0.5 µg/mL, with susceptibility rates of 99.8% and 99.1% according to CLSI and EUCAST criteria, respectively [50]. Corresponding values for CRE were MIC50/MIC90 values of 0.5/4 µg/mL and susceptibility rates of 98.2% (CLSI) and 87.6% (EUCAST) [50] (Table 1). No regional differences were found in susceptibility rates between the USA and Europe for Enterobacterales. The majority of CRE were CR K. pneumoniae [50]. Cefiderocol susceptibility rates of isolates resistant to ceftazidime–avibactam, imipenem–relebactam and meropenem–vaborbactam were 89.2%, 95.9% and 95.1%, respectively, according to CLSI criteria, and 54.1%, 69.4% and 70.7%, respectively, according to EUCAST criteria [50].
Susceptibility rates to cefiderocol were determined against a collection of CRE from two national reference centres in France and Belgium, representing part of the CR Gram-negative isolates in these two countries. Cefiderocol susceptibility rates against 222 CRE isolates based on CLSI and EUCAST breakpoints were 93% and 81%, respectively. The susceptibility rate varied by the expressed carbapenemase enzyme between 81 and 100% by CLSI and between 48 and 92% by EUCAST breakpoints (Table 1) [104].
Cefiderocol Resistance Mechanisms in Enterobacterales: On-Therapy Emergent and Pre-existing Resistance
Mechanisms that can reduce cefiderocol susceptibility in Enterobacterales have been recently reviewed [105] and may include enhanced production of some beta-lactamases (especially NDM and some KPC variants among carbapenemases), modification of siderophore uptake systems, and rarely mutations in PBP targets. When present alone, these mechanisms modestly increase MICs and are usually not sufficient to confer clinically relevant resistance, especially when considering the higher CLSI susceptibility breakpoint of 4 µg/mL. Their combination, however, can eventually increase cefiderocol MICs beyond the clinical breakpoint for resistance [105].
There are a number of reports on the emergence of resistance to cefiderocol. These reports describe resistance either associated with cefiderocol exposure in clinical settings or in vitro (in serial passage experiments), or without cefiderocol exposure in isolates with resistance to other beta-lactams, such as ceftazidime or cefepime, following prior treatment with these agents (Table 2).
To date, two case reports have been published on cefiderocol resistance emerging during therapy [86, 87]. According to these reports, in NDM-producing CRE, cefiderocol exposure may select for mutations in the siderophore receptor cirA gene, or increased NDM enzyme production, leading to cefiderocol resistance [86, 87]. One of these two patients had an NDM- and OXA-48-producing E. cloacae and the second patient had an NDM-5-producing E. coli; both patients had serious comorbidities (i.e., liver transplantation and acute myeloid leukaemia) [86, 87]. The patient with NDM-producing E. cloacae had IAI and BSI, and, although follow-up blood samples became negative, the bile samples remained positive for E. cloacae after 21 days of cefiderocol treatment, and the isolates tested resistant to cefiderocol (MIC > 256 µg/mL) with cirA mutations [86]. The second patient received 19 days of cefiderocol treatment; the mutant E. coli isolates resistant to cefiderocol had an increased copy number and expression of NDM-5 gene and also, in some cases, mutations in the envZ gene [87]. In vitro serial passage experiments in NDM-producing K. pneumoniae strains resulted in similar findings; however, loss of fitness was also described for the mutant strains [106, 107].
While on-therapy, ≥ 4-fold increases in MIC occurred in seven Enterobacterales isolates in cefiderocol-treated patients and in seven isolates from patients in the control arms of two randomised clinical studies (i.e., cefiderocol MICs increased in cefiderocol-treated patients, while meropenem or BAT agents MICs increased in meropenem- or BAT-treated patients in APEKS-NP and CREDIBLE-CR studies, respectively) [82, 108, 109]. Cefiderocol resistance developed in only one E. cloacae isolate based on EUCAST criteria and had intermediate susceptibility based on CLSI and FDA criteria. In this isolate a mutation in the gene for the ACT-17 enzyme had occurred, and cloning experiments in E. coli confirmed the role of this mutation in increasing cefiderocol MIC twofold [108]. The other six post-treatment isolates with ≥ 4-fold increases in cefiderocol MIC remained susceptible according to EUCAST, CLSI, and FDA criteria [108]. No mutations in iron transport-related genes were found [108]. In the control arms, six of seven isolates became resistant to the antibiotic used for treatment [82, 109].
There are some reports on cefiderocol resistance without prior drug exposure. Shields et al. described a patient without previous exposure to cefiderocol who had necrotising pancreatitis and septic shock, complicated by ventilator-associated pneumonia (VAP), who received piperacillin-tazobactam, meropenem (23 days), cefepime (11 days), and ceftazidime–avibactam (1 day) sequentially [110]. E. cloacae-hormaechei isolates developed mutations in porin channels leading to carbapenem resistance and a mutation in ampC (deletion of two amino acids) that conferred resistance to cefepime, ceftazidime–avibactam and cefiderocol. Neither carbapenemases nor ESBLs were present in parental and mutant E. cloacae isolates. It was postulated that the identified A292_L293del AmpC mutation was responsible for resistance to the cephalosporin class rather than one agent alone [110]. In another case, Kawai et al. showed that E. cloacae with reduced susceptibility or resistance to ceftazidime–avibactam and cefiderocol harboured a mutant form of AmpC with deletion of two amino acids, which significantly altered the structure of the enzyme in the R2 loop, leading to increased hydrolysis of these antibiotics [88]. The hydrolytic efficiency towards ceftazidime increased ~ 1000-fold, whereas, for cefiderocol, it was increased by ~ 20-fold. It was postulated that cefiderocol resistance with an MIC value of > 16 µg/mL could be the result of increased expression of mutant AmpC in E. cloacae [88].
Tiseo et al. showed that a mutation in the gene encoding the KPC-3 enzyme resulted in a CR KPC-K. pneumoniae isolate developing resistance to ceftazidime–avibactam (following exposure) and cefiderocol (without exposure to cefiderocol), while restoring susceptibility to meropenem and imipenem [84]. Similar findings were reported by Bianco et al. [111]. In their investigation, following treatment of patients with ceftazidime–avibactam, mutations in KPC were detected among ceftazidime–avibactam-resistant KPC-producing CRE, and a high rate of cefiderocol resistance was also observed [111]. In contrast, these isolates demonstrated impaired carbapenemase activity and low rates of carbapenem resistance [111]. Among ceftazidime–avibactam-susceptible KPC-producing isolates, the cefiderocol susceptibility rate was high, whereas the carbapenem susceptibility rate was low [111].
The role of mutations in KPC in the development of cefiderocol resistance was investigated in additional in vitro studies. Poirel et al. showed that two different mutations in KPC-3 in CR K. pneumoniae strains, resulting in KPC-41 and KPC-50 variants under in vitro antibiotic pressure, had cross-resistance between ceftazidime–avibactam and cefiderocol based on EUCAST breakpoints [112]. These CR K. pneumoniae variants had mutations in porin genes; however, susceptibility to meropenem was restored [112]. Hobson et al. showed that CR KPC-K. pneumoniae with mutations in KPC had increased cefiderocol MIC (highest MIC, 4 µg/mL) and developed resistance to ceftazidime–avibactam. All isolates growing at a high inoculum level had cefiderocol resistance (MICs 4 to > 32 µg/mL) [113].
Jacob et al. showed that a recently identified OXA variant (OXA-427) in Enterobacterales conferred cefiderocol resistance (zone diameters 5–15 mm), although the exact mechanism has not been confirmed [114]. Mc Gann et al. found an NDM- and Pseudomonas extended-resistant (PER) beta-lactamase-producing pan-drug-resistant Providencia rettgeri that was resistant to several antibiotics, including cefiderocol [115].
Price et al. identified an NDM-5-producing CR E. coli strain that was resistant to cefiderocol without prior exposure to cefiderocol (zone diameter 7 mm) [116]. Comparing this strain with other genetically similar (equivalent sequence type, NDM-producing) cefiderocol-susceptible and cefiderocol-resistant CR E. coli strains, the authors suggested that a mutation in the cirA gene (via truncation and premature stop codon) was likely to be responsible for cefiderocol resistance [116]. Similar findings were reported on the role of NDM enzyme by Lan et al. [117] and Jousset et al. [118]. Previous work by Ito et al. suggested that E. coli isolates with mutations in cirA and fiu genes resulted in a 16-fold increase in cefiderocol MIC values, although the isolates remained susceptible [92]. Sato et al. also showed that YRIN mutations in PBP-3 may contribute to reduced susceptibility to cephalosporins and monobactams [119]. The observations on the role of NDM enzyme, cirA mutation, and YRIN insertion were similar to those by the in vitro susceptibility studies conducted in China on 1158 CRE isolates without prior exposure to cefiderocol [120] and in Switzerland in one CR E. coli isolate [121], showing resistance to cefiderocol when these mechanisms are jointly present. These investigations describing cefiderocol-resistant NDM-producing CRE suggest that such strains emerged prior to clinical use of cefiderocol. Moreover, a nosocomial outbreak of NDM-producing K. pneumoniae resistant to cefiderocol, mostly caused by a mutant clone with a cirA mutation, was recently reported. The resistant clone was able to spread in the absence of significant selective pressure by cefiderocol, suggesting that similar mutants do not necessarily exhibit a fitness defect [122]. Infection control to prevent outbreaks linked to such strains is essential to preserve the activity and utility of cefiderocol for the treatment of CRE infections [122, 123].
Simner et al. investigated in detail the potential mechanisms of cefiderocol resistance in 56 CRE using whole-genome sequencing and found that isolates with increased cefiderocol MIC values had a heterogeneous mechanism profile [124]. Fourteen isolates (25%) with cefiderocol MICs ≥ 4 µg/mL were found, but no consistent mechanism across these isolates could be confirmed. The expression of beta-lactamases, including carbapenemases, in combination with permeability defects might have explained the increased cefiderocol MICs [124].
It is worth noting that the addition of both the serine beta-lactamase inhibitor, avibactam, and the MBL inhibitor, dipicolinic acid, reduced the MICs of cefiderocol against previously non-susceptible Enterobacterales isolates [125, 126].
Pharmacokinetics/Pharmacodynamics, and Effectiveness of Cefiderocol in Preclinical Models
Early pharmacokinetic and pharmacodynamic (PK/PD) preclinical studies in various infection models have established that cefiderocol is a time-dependent cephalosporin, and that the fraction of time (i.e., dosing period) that the unbound drug concentration exceeds the MIC (% fT>MIC) is the PD driver of its antibacterial effect in vivo. The preclinical dose-finding experiments in murine models supported the administration of cefiderocol 2 g every 8 h in 3-h infusions, which achieved bactericidal effects against Gram-negative bacteria with cefiderocol MIC values up to 4 µg/mL [127]. The in vivo efficacy of cefiderocol against E. coli and K. pneumoniae has been demonstrated in neutropenic murine thigh infection and lung infection models, as well as in an immunocompetent rat lung infection model and in an in vitro chemostat model [128,129,130,131,132].
Cefiderocol administration at humanised dosing achieved stasis or a ≥ 1-log10 reduction in bacterial density against CR E. coli and CR K. pneumoniae [128]; this was achieved against 77% of tested Enterobacterales strains, which had cefiderocol MIC values up to ≤ 4 µg/mL. Growth of Enterobacterales strains with cefiderocol MIC values of ≥ 8 µg/mL was not inhibited in this model [128]. Similar inhibitory effects were shown by Stainton et al. in a murine thigh infection model against AmpC-producing E. coli and CR KPC-producing K. pneumoniae strains for up to 72 h [129]. Nakamura et al. demonstrated that a 1-log10 reduction in bacterial density would be achieved at ~ 73% fT>MIC in a murine thigh infection model and at ~ 64% fT>MIC in a murine lung infection model, suggesting a PD target of ~ 75% fT>MIC for Enterobacterales infections [130]. In a rat lung infection model, humanised dosing of cefiderocol, simulating 3-h infusions every 8 h, resulted in bactericidal effects against both KPC-producing CR K. pneumoniae and NDM-producing K. pneumoniae [131]. In these experiments, one NDM-producing CR K. pneumoniae strain had a cefiderocol MIC of 8 µg/mL; however, reduction in bacterial growth was achieved [131].
Further investigations in the in vitro chemostat model confirmed the bactericidal activity of cefiderocol with humanised dosing, 2 g every 8 h as a 3-h infusion, against CR K. pneumoniae and CR E. coli producing carbapenemases (NDM, KPC), ESBLs (SHV-11, CTX-M-15) and acquired AmpC (CMY-2) enzymes, with cefiderocol MIC values of 2–4 µg/mL [132].
To justify the dosing recommendation from the preclinical studies with CR infections, Monte-Carlo simulations for various patient populations were performed and showed that cefiderocol 2 g every 8 h over a 3-h infusion period would reach a > 90% probability of target attainment (PTA) to achieve 75% fT>MIC for isolates with cefiderocol MIC values up to 4 µg/mL [133]. The optimised population PK modelling following incorporation of PK data from Phase 3 clinical studies from patients with NP, BSI/sepsis and cUTI confirmed that cefiderocol 2 g every 8 h in a 3-h infusion provided > 90% PTA for 100% fT>MIC against MICs of ≤ 4 μg/mL for all infection sites across all renal function groups, including patients with augmented renal clearance, except for patients with BSI and normal renal function (> 85% PTA) [134].
Cefiderocol demonstrated a linear PK profile at ascending doses, and its plasma levels correlate with renal function [135,136,137,138]. Renal impairment decreases its clearance and increases the plasma area under the concentration-time curve; therefore, dose adjustment is recommended for patients with renal impairment, including patients with continuous renal replacement therapy [138, 139]. A Phase 1b study in pneumonia patients who underwent broncho alveolar lavage to estimate lung penetration of cefiderocol following multiple doses demonstrated that cefiderocol penetrates the epithelial lining fluid (ELF) [81]. Application of PK modelling in seven patients with nosocomial pneumonia who received cefiderocol 2 g every 8 h in a 3-h infusion reported ≥ 99.5% PTA for 100% fT>MIC,ELF against MICs of ≤ 2 μg/mL and ≥ 87.0% against MICs of ≤ 4 μg/mL regardless of renal function [140].
Clinical Efficacy of Cefiderocol
To date, three prospective, randomised clinical studies have been conducted in a total of 906 patients. The efficacy and safety of cefiderocol was investigated in infections caused by Enterobacterales in > 350 patients with cUTI, NP and BSI/sepsis across three randomised controlled studies (i.e., CREDIBLE-CR, APEKS-NP, and APEKS-cUTI) [82, 109, 141]. Most patients with Enterobacterales were enrolled in the double-blind Phase 2 APEKS-cUTI study, in which E. coli (60.3%) and K. pneumoniae (19.0%) were the two most frequent species [141], and only a small proportion had CRE infections receiving cefiderocol [(2.4% (6/252)] [142]. The APEKS-cUTI study was not designed to enrol patients with CR infections as imipenem–cilastatin was the comparator agent. However, patients had underlying risk factors for multidrug-resistant Gram-negative pathogens [141]. The overall results of the APEKS-cUTI study showed that the composite endpoint of clinical response and microbiological response at test of cure was achieved in 73% of patients in the cefiderocol arm versus 55% of patients in the imipenem–cilastatin arm [adjusted difference 18.6%, 95% confidence interval (CI) 8.2; 28.9] [141]. Similar results were found for patients with E. coli (cefiderocol 74%, imipenem–cilastatin 58%) and K. pneumoniae (cefiderocol 74%, imipenem–cilastatin 48%) (Table 3) [141]. Among six patients with NDM- and/or OXA-48-producing CR K. pneumoniae, clinical cure (100%) and microbiological eradication (83.3%) rates at test of cure were high in cefiderocol-treated patients (Table 3) [142]. Only one patient had CR KPC-producing K. pneumoniae in the imipenem–cilastatin arm, and this patient achieved clinical cure and eradication by test of cure. None of the patients with CRE cUTI died in this study [142].
Patients with suspected Gram-negative bacterial NP were randomised in the double-blind, controlled, non-inferiority Phase 3 APEKS-NP study to receive either cefiderocol 2 g every 8 h in 3-h infusions or high-dose, extended-infusion meropenem for 7–14 days [109]. Among 292 pneumonia patients in the modified intention-to-treat population, 58.6% (85/145) and 58.5% (86/147) had Enterobacterales identified as a pathogen in the cefiderocol and meropenem arms, respectively, with K. pneumoniae being the most frequent species, followed by E. coli and E. cloacae in both arms [109]. This study was not designed to enrol patients with CR pathogens; however, 16.5% (14/85) and 8.1% (7/86) had at least one CRE at randomisation in the cefiderocol and meropenem arms, respectively [142]. The CRE isolates in this study expressed a variety of beta-lactamases, including ESBLs, NDM and OXA-48 carbapenemases. In these CRE isolates, porin mutations were also detected and some isolates were non-CP CRE [142]. The study met the overall primary endpoint of non-inferiority for all-cause mortality (ACM) at Day 14 [109]. Among patients with pneumonia caused by K. pneumoniae and E. coli overall, comparable clinical cure (cefiderocol 65% and 63%, meropenem 66% and 59%, respectively) and microbiological eradication (cefiderocol 44% and 53%, meropenem 50% and 50%, respectively) rates were achieved (Table 3) [109]. Among patients with CRE infections, clinical cure [cefiderocol 57.1% (8/14), meropenem 71.4% (5/7)] was observed in similar proportion of patients, although these patient numbers were relatively small [142] (Table 3). For Enterobacterales infections, the overall ACM rates at Day 14 [cefiderocol 12%, meropenem 10% (treatment difference 1.1%, 95% CI −9.4; 11.7) and at Day 28 [cefiderocol 22%, meropenem 19% (treatment difference 2.7, 95% CI −11.0; 16.3)] were similar between treatment arms [109]. Among patients with CRE pneumonia by Day 28, three died in the cefiderocol arm and one died in the meropenem arm [142].
The third clinical study was specifically designed to enrol patients with infections caused by CR Gram-negative pathogens, including CRE, and only a limited number of exclusion criteria were specified in order to facilitate patient enrolment. The pathogen-focused, open-label, randomised (2:1), descriptive Phase 3 CREDIBLE-CR study assessed the efficacy of cefiderocol, 2 g every 8 h in a 3-h infusion, and BAT in 150 hospitalised patients with serious CR Gram-negative infections [82]. Nearly half (45%) of the patient population had NP, 25% had BSI/sepsis and 30% had cUTI. Most patients received cefiderocol in monotherapy, and the majority of patients in the BAT arm received colistin-based combination therapy [82]. The primary endpoint was clinical cure in patients with NP and BSI/sepsis, and microbiological eradication in patients with cUTI. The overall clinical cure rates were 52.5% (42/80) for cefiderocol and 50.0% (19/38) for BAT. Clinical cure rates at test of cure in the cefiderocol and the BAT arms were: 50.0% (20/40) and 52.6% (10/19), respectively, in patients with pneumonia; 43.5% (10/23) and 42.9% (6/14), respectively, in patients with BSI/sepsis; and 70.6% (12/17) and 60.0% (3/5), respectively, in patients with cUTI [82]. A range of molecular mechanisms behind carbapenem resistance, often in parallel, were present in CRE, and included expression of KPC, OXA-48, NDM, OXA-48 + NDM, VIM carbapenemases, porin channel mutations and expression of ESBLs [47, 142, 143]. For CRE infections across all diagnoses, the overall clinical cure rate was 66% with cefiderocol and 45% with BAT, and the microbiological eradication rate was 48% with cefiderocol and 18% with BAT (Table 3). In the subset of patients with CRE pneumonia, 62.5% (5/8) of patients with cefiderocol and 37.5% (3/8) of patients with BAT had clinical cure [142]. In the subgroup of patients with cUTI caused by CRE, 83.3% (10/12) of patients with cefiderocol and 66.7% (2/3) with BAT had clinical cure [142]. ACM rates at Day 28 in cefiderocol-treated patients with NP [cefiderocol 12.5% (1/8); BAT 25.0% (2/8)] or cUTI (cefiderocol 8.3%; BAT 33.3%) remained low [142]. Across the APEKS-NP and CREDIBLE-CR studies, 10 patients had OXA-48-positive Enterobacterales isolates, all of which were K. pneumoniae. Clinical cure was achieved in seven of these patients, with one clinical failure and two indeterminate outcomes [144].
A post-hoc analysis of 84 patients with bacteraemia enrolled into the APEKS-cUTI, APEKS-NP and CREDIBLE-CR studies demonstrated that cefiderocol eradicated the baseline pathogens, including CRE, by days 3–4 in a large proportion of patients, and persistence or recurrence rates were low [143]. A total of 62 Enterobacterales isolates were grown from blood cultures across the three studies. Most CRE blood isolates were identified in the CREDIBLE-CR study, including patients with BSI/sepsis, NP and cUTI. The highest eradication rates of Enterobacterales with cefiderocol treatment [100% (18/18)] was found in the APEKS-cUTI study. In the CREDIBLE-CR study, within 3–4 days, 75.0% (9/12) of CRE were eradicated with cefiderocol treatment and 50.0% (3/6) with BAT. This analysis also showed that eradication rates at this early time point were similar by various resistance mechanisms [75.0% (3/4) for MBL-producing CRE, 75.0% (6/8) for serine-carbapenemase-producing CRE, and 71.4% (5/7) for ESBL-producing CRE] [143]. Corresponding clinical cure rates at test of cure were 75.0% (3/4), 50.0% (4/8) and 57.1% (4/7), respectively, in the cefiderocol arm [143].
In another post hoc analysis, looking solely at infections caused by MBL-producing pathogens in the CREDIBLE-CR and APEKS-NP studies, most CRE expressed an NDM enzyme, and a smaller proportion expressed a VIM enzyme [47]. These isolates were highly resistant to meropenem. Cefiderocol treatment eradicated MBL-expressing CRE in 7 of 10 patients by the end of treatment, and 8 of 10 patients had clinical cure at test of cure in the CREDIBLE-CR study [47] (Table 3). In the APEKS-NP study, 60% of the MBL-producing CRE were eradicated and 60% of patients had clinical cure (Table 3). In a case series of 18 Italian patients with MBL-producing CRE infections, who were treated with cefiderocol in combination therapy, the clinical cure rate (72.2%) at test of cure, the eradication rate (77.8%) at end of cefiderocol treatment and the ACM rate (22.2%) at Day 28 (Table 3) were similar to the outcomes in cefiderocol-treated patients in the analysis by Timsit et al. [47, 145]. Most CRE in the case series expressed NDM, and 76% of patients received cefiderocol in combination therapy [145].
In addition to case reports of resistance [86, 87], eight case reports of CRE infections treated with cefiderocol have been published. Cefiderocol treatment for 14 days resulted in successful eradication of an OXA-48+NDM-1-producing CR K. pneumoniae in a patient with BSI, although the patient later died due to hospital-acquired pneumonia [146]. Cefiderocol treatment for 14 days in a patient with VAP and BSI led to a successful outcome in a polymicrobial infection of extensively drug-resistant A. baumannii and KPC-producing CR K. pneumoniae [147]. In the compassionate use programme, patients with CR Gram-negative pathogens and life-threatening conditions were treated with cefiderocol. Thus, patients with osteomyelitis or prosthetic joint infection caused by pathogens that lack active treatment options were treated long term. In two such cases, (1) ESBL- and ACT-5-producing E. hormaechei, and (2) ESBL-positive K. pneumoniae with extensively drug-resistant P. aeruginosa, cefiderocol treatment given for 10 and 14 weeks resulted in successful outcomes [148, 149]. However, for one patient with respiratory tract infection due to OXA-48-producing K. pneumoniae with NDM-1-producing P. aeruginosa, cefiderocol treatment did not result in a favourable outcome [150]. Furthermore, a diabetic kidney transplant patient, who was infected by a CR K. pneumoniae expressing NDM-1 and OXA-232 enzymes, had a fatal outcome despite eradication of the blood isolate with cefiderocol-containing combination treatment [151]. According to a recent case report of a 44-year-old male patient, who developed meningitis due to KPC-producing K. pneumoniae, 14-day treatment with cefiderocol plus trimethoprim-sulfamethoxazole resulted in eradication of K. pneumoniae from the cerebrospinal fluid and clinical success [152]. In a paediatric case report of BSI caused by VIM-producing K. pneumoniae, cefiderocol treatment resulted in clinical improvement and clearance of the blood isolate, although paediatric dosing for cefiderocol has yet to be established in clinical studies [153].
Discussion
Since the latest call by the WHO for urgent development of new antibiotics against the priority pathogens, CR A. baumannii, CR P. aeruginosa and CRE, research has been intensified, with some success. Despite the latest approvals of new antibiotics, such as ceftazidime–avibactam, cefiderocol, meropenem–vaborbactam, imipenem–relebactam, eravacycline and plazomicin, more clinical research is needed to establish their role in the management of CRE infections. Pathogen-focused descriptive clinical studies with these new antibiotics, which prospectively enrolled patients with CRE infections, have found clinical cure (59.4–65.5%, except plazomicin 35.3%) and ACM (11.8–15.6%) rates that were relatively similar across studies [73, 74, 82, 83]. Of note, these new agents were administered in monotherapy in these clinical studies and comparators were frequently colistin-based regimens. Only one retrospective study compared two new agents head-to-head (i.e., ceftazidime–avibactam and meropenem–vaborbactam) [154]. Data on imipenem–relebactam are very limited in CRE infections (i.e., only six patients with CRE in RESTORE-IMI 1), although the RESTORE-IMI 2 study enrolled 178 patients with Enterobacterales pneumonia [72]. Nevertheless, these are notable outcomes for the new antibiotics compared with historical mortality rates of more than 40% [28, 29].
Enterobacterales frequently express ESBLs, which are responsible for resistance to extended spectrum cephalosporins. For the treatment of critically ill patients with severe infections, carbapenems are the preferred option. Previously hospitalised patients with recurrent infections often require alternative treatment options, as prior carbapenem use and use of indwelling catheters are risk factors for colonisation and subsequent infections by CRE. Similarly, patients repatriated or having visited countries with high rates of CP Gram-negative pathogens are at increased risk for carriage and thus infections by these pathogens. For such patients and for those who are critically ill or have comorbidities that may be associated with poor outcomes, such as immunosuppression, cancer or renal impairment, the newer agents are preferred. The older antibiotics, such as colistin, amikacin and tigecycline, are known to increase the risk of toxicity or are associated with inadequate plasma levels, as reported in several studies [155,156,157,158]. The safety profile of the newer agents is a key benefit for the treatment of patients with CRE infections. Beta-lactams are preferred over polymyxins or aminoglycosides, which may be nephrotoxic [159].
The recent IDSA guidance and ESCMID guidelines provide recommendations on when and how to use the new agents [89, 90]. Neither of them recommends a second agent to be used in combination with the new antibiotics for the treatment of CRE infections. Resistance has been reported for each of the new agents; however, these agents still have high susceptibility rates globally, with some regional variations. Overall susceptibility rates are reduced for ceftazidime–avibactam, meropenem–vaborbactam, and imipenem–relebactam in regions where MBLs are prevalent, while cefiderocol MICs are higher where NDM-producing CRE are more prevalent, but many MBL producers remain susceptible to it. Rapid molecular diagnostic testing for resistance mechanisms should lead to the improved surveillance and diagnosis of CRE and, hence, to the selection of the most appropriate antibiotic agent [160]. Indeed, in addition to the recommendations [89, 90], rapid diagnostics is one of the issues that will guide the optimal use of newer antibiotics against CRE. Other considerations include those related to the hospital formulary (such as local epidemiology and drug acquisition costs) and the development of criteria for use, as part of antimicrobial stewardship [161]. Another major limiting factor for the treatment of infections caused by CRE may be a lack of availability of newer agents along with susceptibility testing methods in countries with locally relevant enzymatic epidemiology, the very regions where these agents may be of most benefit.
The most therapeutically challenging carbapenemases are the MBLs, among which NDM, VIM and IMP (in South-East Asia) enzymes are the MBLs most commonly produced by CRE. None of the new beta-lactam–beta-lactamase inhibitor agents alone has activity; the combination of ceftazidime–avibactam with aztreonam, even in the absence of in vitro activity separately, may be effective [162]. Aztreonam–avibactam combination is currently under Phase 3 clinical development and may be available in the future [163].
In addition to aztreonam–avibactam, cefiderocol is the only other beta-lactam with activity against MBL-producing CRE. In vitro studies and surveillance studies showed that cefiderocol MIC values are higher against NDM-producing isolates than VIM-producing isolates. Nevertheless, clinical studies demonstrated that NDM-producing CRE infections with cefiderocol MICs of 4 µg/mL, which corresponds to the CLSI susceptibility breakpoint, could be successfully treated with cefiderocol [47]. Based on resistance reports, an increased copy number of NDM may increase cefiderocol MIC values beyond the resistance breakpoint [87]. Moreover, mutations affecting siderophore uptake systems (especially the CirA siderophore receptor) can confer high-level resistance to cefiderocol in NDM-producing strains [86]. Emergence of these mutants has been occasionally reported under cefiderocol treatment [86, 87], and was also observed in vitro, although loss of fitness was observed in these isolates [107]. Of note, the combination of mutations in iron transport genes and expression of NDM enzyme was found in CRE in China, where cefiderocol has not been used in clinical practice [117, 120]; thus, resistance to cefiderocol by this mechanism may occur as a collateral damage driven by other, so far unknown mechanisms of selection [113].
In CRE, the development of resistance to cefiderocol treatment appears infrequent and, based on case reports, no consistent mechanisms or mutations are associated with reduced cefiderocol susceptibility. To date, cefiderocol resistance following treatment was demonstrated for two patients in case reports and one patient in Phase 3 clinical studies [82, 108, 109]. Of note, pre-existing resistance to cefiderocol, without exposure to the drug, has been described in some case reports. These patients were treated with other beta-lactam antibiotics and developed resistance to ceftazidime–avibactam with cross-resistance to cefiderocol as a result of certain mutations emerging in KPC-2, KPC-3 or AmpC [84, 88, 110]. Cross-resistance may emerge for the cephalosporin class with specific mutations in AmpC [88, 110]. The combination of the expression of the NDM enzyme and mutation in the cirA gene has been reported in other case reports of cefiderocol resistance [86]. However, NDM MBLs or cirA mutations have not been reported in cases with KPC or AmpC mutations. Thus, the reports suggest that, rather than a single mutation causing resistance, the presence of different mechanisms or mutations in parallel is necessary to develop clinically relevant cefiderocol resistance [87, 105].
The efficacy of cefiderocol in monotherapy was established in a large number of patients with carbapenem-susceptible Enterobacterales infections in two randomised controlled trials, and in a smaller number of patients with CRE in the CREDIBLE-CR study. The mortality rates with cefiderocol treatment in patients with CRE infections were similar to those seen in other pathogen-focused clinical studies with other newer antibiotics [83]. One limitation of all these pathogen-focused studies was the relatively low number of randomised patients [83]. In patients with CRE infections in CREDIBLE-CR, an overall benefit was seen with cefiderocol treatment. In cUTI patients with CRE, high rates of clinical and microbiological favourable cure rates were seen in both APEKS-cUTI and CREDIBLE-CR studies. In pneumonia patients, the clinical cure rates were similar in APEKS-NP (57%) and CREDIBLE-CR studies (62%), and these rates were also within the range seen for patients with carbapenem-susceptible Enterobacterales (64% and 56%, respectively) and in other pneumonia studies for Enterobacterales (RESTORE-IMI 2: imipenem–relebactam 61%, piperacillin-tazobactam 55.8% [72)]. Early eradication of CRE harbouring Class A ESBLs, MBLs and other carbapenemases was confirmed from blood samples with cefiderocol treatment in patients with bacteraemia, and persistence and recurrence rates were low [143].
Conclusions
Cefiderocol is a potent beta-lactam (siderophore) antibiotic against CRE producing serine- or metallo-carbapenemases, ESBLs and/or AmpC enzymes. As a siderophore cephalosporin, cefiderocol has a unique and efficient mode of cell entry in Gram-negative pathogens, which may overcome resistance mechanisms involving alterations of porin channels and upregulation of efflux pumps. The efficacy and safety of cefiderocol was demonstrated in patients infected by various Enterobacterales species, including CRE producing KPC, MBL or OXA-48-type enzymes, across different infection sites. There is a need for additional comparative effectiveness clinical data to establish the value of cefiderocol in the management of CRE infections for seriously ill patient populations. Furthermore, continued surveillance is imperative to monitor emergence of resistance under cefiderocol treatment among pathogens in the clinical setting.
References
Antimicrobial Resistance Collaborators (ARC). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55.
WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022: 2020 data. Copenhagen: WHO Regional Office for Europe; 2022. https://www.ecdc.europa.eu/sites/default/files/documents/ECDC-WHO-AMR-report.pdf. Accessed 9 March 2022.
World Health Organisation. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; 2021. https://www.who.int/publications/i/item/9789240027336. Accessed: 9 March 2022.
Gao Y, Chen M, Cai M, et al. An analysis of risk factors for carbapenem-resistant Enterobacteriaceae infection. J Glob Antimicrob Resist. 2022;30:191–8.
Jean SS, Harnod D, Hsueh PR. Global threat of carbapenem-resistant Gram-negative bacteria. Front Cell Infect Microbiol. 2022;12: 823684.
Wang M, Earley M, Chen L, Multi-Drug Resistant Organism Network Investigators, et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): a prospective, multicentre, cohort study. Lancet Infect Dis. 2022;22(3):401–12.
van Duin D, Arias CA, Komarow L, Multi-Drug Resistant Organism Network Investigators, et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis. 2020;20(6):731–41.
Lin Q, Wu M, Yu H, et al. Clinical and microbiological characterization of carbapenem-resistant Enterobacteriales: a prospective cohort study. Front Pharmacol. 2021;12: 716324. https://doi.org/10.3389/fphar.2021.716324.
Ambretti S, Bassetti M, Clerici P, et al. Screening for carriage of carbapenem-resistant Enterobacteriaceae in settings of high endemicity: a position paper from an Italian working group on CRE infections. Antimicrob Resist Infect Control. 2019;8:136.
Palacios-Baena ZR, Giannella M, Manissero D, et al. Risk factors for carbapenem-resistant Gram-negative bacterial infections: a systematic review. Clin Microbiol Infect. 2021;27(2):228–35.
Chen Y, Wang WL, Zhang W, et al. Risk factors and outcomes of carbapenem-resistant Enterobacteriaceae infection after liver transplantation: a retrospective study in a Chinese population. Infect Drug Resist. 2020;13:4039–45.
Freire MP, Carvalho LB, Reusing JO Jr, et al. Carbapenem-resistant Enterobacteriaceae among kidney transplant recipients—insights on the risk of acquisition and CRE infection. Infect Dis (Lond). 2021;53(6):430–9.
Bouganim R, Dykman L, Fakeh O, et al. The clinical and molecular epidemiology of noncarbapenemase-producing carbapenem-resistant Enterobacteriaceae: a case-case-control matched analysis. Open Forum Infect Dis. 2020;7(8): ofaa299.
Lin MY, Ray MJ, Rezny S, Runningdeer E, Weinstein RA, Trick WE. Predicting carbapenem-resistant Enterobacteriaceae carriage at the time of admission using a statewide hospital discharge database. Open Forum Infect Dis. 2019;6(12): ofz483.
Sexton ME, Bower C, Jacob JT. Risk factors for isolation of carbapenem-resistant Enterobacterales from normally sterile sites and urine. Am J Infect Control. 2021;50(8):929–33.
Aleidan FAS, Alkhelaifi H, Alsenaid A, et al. Incidence and risk factors of carbapenem-resistant Enterobacteriaceae infection in intensive care units: a matched case–control study. Expert Rev Anti Infect Ther. 2021;19(3):393–8.
Salomão MC, Freire MP, Boszczowski I, Raymundo SF, Guedes AR, Levin AS. Increased risk for carbapenem-resistant Enterobacteriaceae colonization in intensive care units after hospitalization in emergency department. Emerg Infect Dis. 2020;26(6):1156–63.
O’Hara LM, Nguyen MH, Calfee DP, CDC Prevention Epicenters Program, et al. Risk factors for transmission of carbapenem-resistant Enterobacterales to healthcare personnel gloves and gowns in the USA. J Hosp Infect. 2021;109:58–64.
Gomides MDA, Fontes AMS, Silveira AOSM, Matoso DC, Ferreira AL, Sadoyama G. The importance of active surveillance of carbapenem-resistant Enterobacterales (CRE) in colonization rates in critically ill patients. PLoS One. 2022;17(1): e0262554.
Chuah CH, Gani Y, Sim B, Chidambaram SK. Risk factors of carbapenem-resistant Enterobacteriaceae infection and colonisation: a Malaysian tertiary care hospital-based case–control study. J R Coll Physicians Edinb. 2021;51(1):24–30.
Saito S, Hayakawa K, Tsuzuki S, et al. A matched case-case-control study of the impact of clinical outcomes and risk factors of patients with IMP-type carbapenemase-producing carbapenem-resistant Enterobacteriaceae in Japan. Antimicrob Agents Chemother. 2021;65(3): e01483–20.
Khawcharoenporn T, Laichuthai W. Subsequent carbapenem-resistant Enterobacteriaceae (CRE)-associated infections among hospitalized patients with CRE colonization: impact of antibiotic use and other factors. Infect Control Hosp Epidemiol. 2020;41(9):1084–9.
Predic M, Delano JP, Tremblay E, Iovine N, Brown S, Prins C. Evaluation of patient risk factors for infection with carbapenem-resistant Enterobacteriaceae. Am J Infect Control. 2020;48(9):1028–31.
Howard-Anderson JR, Bower CW, Smith G, et al. Carbapenem-resistant Enterobacterales bacteriuria and subsequent bacteremia: a population-based study. Infect Control Hosp Epidemiol. 2021;42(8):962–7.
Aung AH, Kanagasabai K, Koh J, et al. Epidemiology and transmission of carbapenemase-producing Enterobacteriaceae in a health care network of an acute-care hospital and its affiliated intermediate- and long-term-care facilities in Singapore. Antimicrob Agents Chemother. 2021;65(8): e0258420.
Kassem A, Raed A, Michael T, et al. Risk factors and outcomes of patients colonized with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae. Infect Control Hosp Epidemiol. 2020;41(10):1154–61.
Qian Y, Bi Y, Liu S, Li X, Dong S, Ju M. Predictors of mortality in patients with carbapenem-resistant Klebsiella pneumoniae infection: a meta-analysis and a systematic review. Ann Palliat Med. 2021;10(7):7340–50.
Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943–50.
Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–106.
Hong Nguyen M, Shields RK, Chen L, et al. Molecular epidemiology, natural history, and long-term outcomes of multidrug-resistant Enterobacterales colonization and infections among solid organ transplant recipients. Clin Infect Dis. 2022;74(3):395–406.
Kazmierczak KM, Karlowsky JA, de Jonge BLM, Stone GG, Sahm DF. Epidemiology of carbapenem resistance determinants identified in meropenem-nonsusceptible Enterobacterales collected as part of a global surveillance program, 2012 to 2017. Antimicrob Agents Chemother. 2021;65(7): e0200020.
Hovan MR, Narayanan N, Cedarbaum V, Bhowmick T, Kirn TJ. Comparing mortality in patients with carbapenemase-producing carbapenem resistant Enterobacterales and non-carbapenemase-producing carbapenem resistant Enterobacterales bacteremia. Diagn Microbiol Infect Dis. 2021;101(4): 115505.
Black CA, So W, Dallas SS, et al. Predominance of non-carbapenemase producing carbapenem-resistant Enterobacterales in South Texas. Front Microbiol. 2021;11: 623574.
Kazmierczak KM, de Jonge BLM, Stone GG, Sahm DF. Longitudinal analysis of ESBL and carbapenemase carriage among Enterobacterales and Pseudomonas aeruginosa isolates collected in Europe as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance programme, 2013–17. J Antimicrob Chemother. 2020;75(5):1165–73.
Tamma PD, Goodman KE, Harris AD, et al. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis. 2017;64(3):257–64.
Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl. 7):S521–8.
Kois AK, Nicolau DP, Kuti JL. Unresolved issues in the identification and treatment of carbapenem-resistant Gram-negative organisms. Curr Opin Infect Dis. 2020;33(6):482–94.
Khalifa SM, Abd El-Aziz AM, Hassan R, Abdelmegeed ES. β-lactam resistance associated with β-lactamase production and porin alteration in clinical isolates of E. coli and K. pneumoniae. PLoS One. 2021;16(5): e0251594.
Findlay J, Hamouda A, Dancer SJ, Amyes SG. Rapid acquisition of decreased carbapenem susceptibility in a strain of Klebsiella pneumoniae arising during meropenem therapy. Clin Microbiol Infect. 2012;18(2):140–6.
Ambler RP. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289:321–31.
Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–76.
Naas T, Oueslati S, Bonnin RA, et al. Beta-lactamase database (BLDB)—structure and function. J Enzyme Inhib Med Chem. 2017;32(1):917–9.
Bonnin RA, Jousset AB, Emeraud C, Oueslati S, Dortet L, Naas T. Genetic diversity, biochemical properties, and detection methods of minor carbapenemases in Enterobacterales. Front Med (Lausanne). 2021;7: 616490.
Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440–58.
Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012;67:1597–606.
Yamano Y. In vitro activity of cefiderocol against a broad range of clinically important Gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl. 7):S544–51.
Timsit JF, Paul M, Shields RK, et al. Cefiderocol for the treatment of infections due to metallo-beta-lactamase-producing pathogens in the CREDIBLE-CR and APEKS-NP Phase 3 randomized studies. Clin Infect Dis. 2022;75(6):1081–4.
Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22:161–82.
Castanheira M, Doyle TB, Kantro V, Mendes RE, Shortridge D. Meropenem–vaborbactam activity against carbapenem-resistant Enterobacterales isolates collected in U.S. hospitals during 2016 to 2018. Antimicrob Agents Chemother. 2020;64(2):e01951–19 (Erratum in: Antimicrob Agents Chemother. 2020;64(4):e00305–20).
Shortridge D, Streit JM, Mendes R, Castanheira M. In vitro activity of cefiderocol against U.S. and European Gram-negative clinical isolates collected in 2020 as part of the SENTRY Antimicrobial Surveillance Program. Microbiol Spectr. 2022;10: e0271221.
Kawamoto Y, Kaku N, Akamatsu N, et al. The surveillance of colistin resistance and mobilized colistin resistance genes in multidrug-resistant Enterobacteriaceae isolated in Japan. Int J Antimicrob Agents. 2022;59(1): 106480.
Avycaz (ceftazidime–avibactam for injection for intravenous use). Prescribing Information. Madison: Allergan USA, Inc.; 2020.
Zavicefta. Ceftazidime–avibactam (2 g/0.5 g powder for concentrate for solution for infusion). Summary of Product Characteristics. Ringaskiddy: Pfizer Ireland Pharmaceuticals Operations Support Group; 2021.
Vabomere (meropenem and vaborbactam for injection, for intravenous use). Prescribing Information. Lincolnshire: Melinta Therapeutics, LLC; 2020.
Vaborem (meropenem/vaborbactam 1 g/1 g powder for concentrate for solution for infusion). Summary of Product Characteristics. Luxembourg: Menarini International Operations Luxembourg S.A.; 2021.
Recarbrio (imipenem–cilastatin–relebactam for injection for intravenous use). Prescribing Information. Whitehouse Station: Merck & Co., Inc.; 2020.
Recarbrio. Imipenem–cilastatin–relebactam (500 mg/500 mg/250 mg powder for solution for infusion). Summary of product characteristics. Haarlem: Merck Sharp & Dohme B.V.; 2020.
Zemdri (plazomicin) injection for intravenous use. San Francisco: Achaogen; 2018.
Xerava (eravacycline for injection for intravenous use). Prescribing information. Waltham: Tetraphase Pharmaceuticals, Inc.; 2021.
Fetroja (cefiderocol for injection for intravenous use). Prescribing information. Florham Park: Shionogi Inc.; 2021.
Fetcroja. Cefiderocol (1 g powder for concentrate for solution for infusion). Summary of product characteristics. Amsterdam: Shionogi B.V.; 2020.
Reyes S, Nicolau DP. Precision medicine for the diagnosis and treatment of carbapenem-resistant Enterobacterales: time to think from a different perspective. Expert Rev Anti Infect Ther. 2020;18(8):721–40.
Gatti M, Viaggi B, Rossolini GM, Pea F, Viale P. An evidence-based multidisciplinary approach focused at creating algorithms for targeted therapy of BSIs, cUTIs, and cIAIs caused by Enterobacterales in critically ill adult patients. Infect Drug Resist. 2021;14:2461–98.
Gatti M, Viaggi B, Rossolini GM, Pea F, Viale P. An evidence-based multidisciplinary approach focused at creating algorithms for targeted therapy of infection-related ventilator associated complications (IVACs) caused by Enterobacterales in critically ill adult patients. Expert Rev Anti Infect Ther. 2022;20(3):331–52.
Wagenlehner FM, Sobel JD, Newell P, et al. Ceftazidime–avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63(6):754–62.
Carmeli Y, Armstrong J, Laud PJ, et al. Ceftazidime–avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis. 2016;16:661–73.
Torres A, Zhong N, Pachl J, et al. Ceftazidime–avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18(3):285–95.
Mazuski JE, Gasink LB, Armstrong J, et al. Efficacy and safety of ceftazidime–avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62:1380–9.
Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem–vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA. 2018;319:788–99.
Wagenlehner FME, Cloutier DJ, Komirenko AS, EPIC Study Group, et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380(8):729–40.
Sims M, Mariyanovski V, McLeroth P, et al. Prospective, randomized, double-blind, Phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother. 2017;72(9):2616–26.
Titov I, Wunderink RG, Roquilly A, et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 Study). Clin Infect Dis. 2021;73(11):e4539–48.
Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of meropenem–vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther. 2018;7:439–55.
McKinnell JA, Dwyer JP, Talbot GH, CARE Study Group, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med. 2019;380(8):791–3.
Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. 2020;70(9):1799–808.
Karlowsky JA, Hackel MA, Takemura M, Yamano Y, Echols R, Sahm DF. In vitro susceptibility of Gram-negative pathogens to cefiderocol in five consecutive annual multinational SIDERO-WT surveillance studies, 2014 to 2019. Antimicrob Agents Chemother. 2022;66(2): e0199021.
Nicolau DP, Siew L, Armstrong J, et al. Phase 1 study assessing the steady-state concentration of ceftazidime and avibactam in plasma and epithelial lining fluid following two dosing regimens. J Antimicrob Chemother. 2015;70(10):2862–9.
van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime–avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis. 2018;66(2):163–71.
Shields RK, Nguyen MH, Chen L, et al. Ceftazidime–avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8): e00883-17. https://doi.org/10.1128/AAC.00883-17.
Wenzler E, Gotfried MH, Loutit JS, et al. Meropenem-RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob Agents Chemother. 2015;59(12):7232–9.
Katsube T, Nicolau DP, Rodvold KA, et al. Intrapulmonary pharmacokinetic profile of cefiderocol in mechanically ventilated patients with pneumonia. J Antimicrob Chemother. 2021;76(11):2902–5 (Erratum in: J Antimicrob Chemother. 2021 Sep 17).
Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2021;21(2):226–40.
Lodise TP, Bassetti M, Ferrer R, et al. All-cause mortality rates in adults with carbapenem-resistant Gram-negative bacterial infections: a comprehensive review of pathogen-focused, prospective, randomized, interventional clinical studies. Expert Rev Anti Infect Ther. 2022;20(5):707–19.
Tiseo G, Falcone M, Leonildi A, et al. Meropenem–vaborbactam as salvage therapy for ceftazidime–avibactam-, cefiderocol-resistant ST-512 Klebsiella pneumoniae-producing KPC-31, a D179Y variant of KPC-3. Open Forum Infect Dis. 2021;8(6): ofab141.
Papp-Wallace KM, Mack AR, Taracila MA, Bonomo RA. Resistance to novel β-lactam-β-lactamase inhibitor combinations: the “price of progress.” Infect Dis Clin North Am. 2020;34(4):773–819.
Klein S, Boutin S, Kocer K, et al. Rapid development of cefiderocol resistance in carbapenem-resistant Enterobacter cloacae during therapy is associated with heterogeneous mutations in the catecholate siderophore receptor cirA. Clin Infect Dis. 2022;74(5):905–8.
Simner PJ, Mostafa HH, Bergman Y, et al. Progressive development of cefiderocol resistance in Escherichia coli during therapy is associated with increased blaNDM-5 copy number and gene expression. Clin Infect Dis. 2022;75(1):47–54. https://doi.org/10.1093/cid/ciab888.
Kawai A, McElheny CL, Iovleva A, et al. Structural basis of reduced susceptibility to ceftazidime–avibactam and cefiderocol in Enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother. 2020;64(7): e00198-20.
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2022 guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2022;75(2):187–212. https://doi.org/10.1093/cid/ciac268.
Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28(4):521–47.
Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis. 2019;69(Suppl. 7):S538–43.
Ito A, Sato T, Ota M, et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother. 2017;62(1): e01454–17.
European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0; 2022. http://www.eucast.org.
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed. Wayne: CLSI Supplement M100; 2022.
Bonnin RA, Emeraud C, Jousset AB, Naas T, Dortet L. Comparison of disk diffusion, MIC test strip and broth microdilution methods for cefiderocol susceptibility testing on carbapenem-resistant Enterobacterales. Clin Microbiol Infect. 2022;28(8):1156.e1-1156.e5.
European Committee on Antimicrobial Susceptibility Testing. EUCAST warnings concerning antimicrobial susceptibility testing products or procedures. https://www.eucast.org/ast-of-bacteria/warnings. Accessed 6 Oct 2022.
Hackel MA, Tsuji M, Yamano Y, et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother. 2017;61(9): e00093–17.
Hackel MA, Tsuji M, Yamano Y, et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother. 2018;62(2): e01968–17.
Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. In vitro activity of cefiderocol, a siderophore cephalosporin, against Gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015–2016: SIDERO-WT-2015. Int J Antimicrob Agents. 2019;53(4):456–66.
Kazmierczak KM, Tsuji M, Wise MG, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents. 2019;53(2):177–84.
Longshaw C, Tsuji M, Hackel M, Sahm D, Yamano Y. In vitro activity of cefiderocol (CFDC), a novel siderophore cephalosporin, against difficult-to-treat (DTR) Gram-negative bacterial pathogens from the multinational sentinel surveillance study, SIDERO-WT (2014–2017). Open Forum Infect Dis. 2019;6(Suppl 2):S309–10.
Takemura M, Kazmierczak KM, Hackel M, Sahm DF, Echols R, Yamano Y. In vitro activity of cefiderocol against metallo-β-lactamase-producing Gram-negative bacteria collected in North America and Europe between 2014 and 2017: SIDERO-WT-2014-2016 studies. Open Forum Infect Dis. 2020;7(Suppl 1):S643–4.
Longshaw C, Manissero D, Tsuji M, Echols R, Yamano Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC Antimicrob Resist. 2020;2(3): dlaa060.
Oueslati S, Bogaerts P, Dortet L, et al. In vitro activity of cefiderocol and comparators against carbapenem-resistant Gram-negative pathogens from France and Belgium. Antibiotics (Basel). 2022;11(10):1352.
Karakonstantis S, Rousaki M, Kritsotakis EI. Cefiderocol: systematic review of mechanisms of resistance, heteroresistance and in vivo emergence of resistance. Antibiotics. 2022;11:723.
Nurjadi D, Kocer K, Chanthalangsy Q, Klein S, Heeg K, Boutin S. New Delhi metallo-beta-lactamase facilitates the emergence of cefiderocol resistance in Enterobacter cloacae. Antimicrob Agents Chemother. 2022;66(2): e0201121.
McElheny CL, Fowler EL, Iovleva A, Shields RK, Doi Y. In vitro evolution of cefiderocol resistance in an NDM-producing Klebsiella pneumoniae due to functional loss of cirA. Microbiol Spectr. 2021;9(3): e0177921.
Nordmann P, Shields RK, Doi Y, et al. Mechanisms of reduced susceptibility to cefiderocol among isolates from the CREDIBLE-CR and APEKS-NP clinical trials. Microb Drug Resist. 2022;28(4):398–407.
Wunderink RG, Matsunaga Y, Ariyasu M, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2021;21(2):213–25.
Shields RK, Iovleva A, Kline EG, et al. Clinical evolution of AmpC-mediated ceftazidime–avibactam and cefiderocol resistance in Enterobacter cloacae complex following exposure to cefepime. Clin Infect Dis. 2020;71(10):2713–6.
Bianco G, Boattini M, Comini S, et al. In vitro activity of cefiderocol against ceftazidime–avibactam susceptible and resistant KPC-producing Enterobacterales: cross-resistance and synergistic effects. Eur J Clin Microbiol Infect Dis. 2022;41(1):63–70.
Poirel L, Sadek M, Kusaksizoglu A, Nordmann P. Co-resistance to ceftazidime–avibactam and cefiderocol in clinical isolates producing KPC variants. Eur J Clin Microbiol Infect Dis. 2022;41(4):677–80.
Hobson CA, Cointe A, Jacquier H, et al. Cross-resistance to cefiderocol and ceftazidime–avibactam in KPC β-lactamase mutants and the inoculum effect. Clin Microbiol Infect. 2021;27(8):1172.e7-1172.e10.
Jacob AS, Chong GL, Lagrou K, Depypere M, Desmet S. No in vitro activity of cefiderocol against OXA-427-producing Enterobacterales. J Antimicrob Chemother. 2021;76(12):3317–8.
Mc Gann P, Geringer MR, Hall LR, et al. Pan-drug resistant Providencia rettgeri contributing to a fatal case of COVID-19. J Med Microbiol. 2021;70(8): 001406.
Price TK, Davar K, Contreras D, et al. Case report and genomic analysis of cefiderocol-resistant Escherichia coli clinical isolates. Am J Clin Pathol. 2022;157(2):257–65.
Lan P, Lu Y, Chen Z, et al. Emergence of high-level cefiderocol resistance in carbapenem-resistant Klebsiella pneumoniae from bloodstream infections in patients with hematologic malignancies in China. Microbiol Spectr. 2022;10(2): e0008422.
Jousset A, Poignon C, Yilmaz S, et al. Rapid selection of a cefiderocol resistant Escherichia coli producing NDM-5 associated with a single amino-acid substitution in CirA siderophore receptor. J Antimicrob Chemother (accepted manuscript).
Sato T, Ito A, Ishioka Y, et al. Escherichia coli strains possessing a four amino acid YRIN insertion in PBP3 identified as part of the SIDERO-WT-2014 surveillance study. JAC Antimicrob Resist. 2020;2(3): dlaa081.
Wang Q, Jin L, Sun S, et al. Occurrence of high levels of cefiderocol resistance in carbapenem-resistant Escherichia coli before its approval in China: a report from China CRE-Network. Microbiol Spectr. 2022;10(3): e0267021.
Poirel L, Ortiz de la Rosa JM, Sakaoglu Z, Kusaksizoglu A, Sadek M, Nordmann P. NDM-35-Producing ST167 Escherichia coli highly resistant to β-lactams including cefiderocol. Antimicrob Agents Chemother. 2022;66(8): e0031122.
Coppi M, Antonelli A, Niccolai C, et al. Nosocomial outbreak by NDM-1-producing Klebsiella pneumoniae highly resistant to cefiderocol, Florence, Italy, August 2021 to June 2022. Euro Surveill. 2022;27(43):2200795.
Patiño-Navarrete R, Rosinski-Chupin I, Cabanel N, et al. Stepwise evolution and convergent recombination underlie the global dissemination of carbapenemase-producing Escherichia coli. Genome Med. 2020;12(1):10.
Simner PJ, Beisken S, Bergman Y, Ante M, Posch AE, Tamma PD. Defining baseline mechanisms of cefiderocol resistance in the Enterobacterales. Microb Drug Resist. 2022;28(2):161–70.
Kohira N, Hackel MA, Ishioka Y, et al. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J Glob Antimicrob Resist. 2020;22:738–41.
Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. In vitro activity of cefiderocol, a siderophore cephalosporin, against multidrug-resistant Gram-negative bacteria. Antimicrob Agents Chemother. 2020;64(12):e01582-e1620.
Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. Humanized exposures of cefiderocol, a siderophore cephalosporin, display sustained in vivo activity against siderophore-resistant Pseudomonas aeruginosa. Pharmacology. 2018;101(5–6):278–84.
Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of humanized exposures of cefiderocol (s-649266) against a diverse population of Gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother. 2017;61(11): e01022–17.
Stainton SM, Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of humanized cefiderocol exposures over 72 hours against a diverse group of Gram-negative isolates in the neutropenic murine thigh infection model. Antimicrob Agents Chemother. 2019;63(2): e01040–18.
Nakamura R, Ito-Horiyama T, Takemura M, et al. In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother. 2019;63(9): e02031–18.
Matsumoto S, Singley CM, Hoover J, et al. Efficacy of cefiderocol against carbapenem-resistant Gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother. 2017;61(9): e00700–17.
Matsumoto S, Kanazawa S, Sato T, Yamano Y. Activities of cefiderocol with simulated human plasma concentrations against carbapenem-resistant Gram-negative bacilli in an in vitro chemostat model. Antimicrob Agents Chemother. 2020;64(11): e01128–20.
Katsube T, Wajima T, Ishibashi T, Arjona Ferreira JC, Echols R. Pharmacokinetic/pharmacodynamic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, for dose adjustment based on renal function. Antimicrob Agents Chemother. 2017;61(1): e01381–16.
Kawaguchi N, Katsube T, Echols R, Wajima T. Population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses of cefiderocol, a parenteral siderophore cephalosporin, in patients with pneumonia, bloodstream infection/sepsis, or complicated urinary tract infection. Antimicrob Agents Chemother. 2021;65(3): e01437–20.
Saisho Y, Katsube T, White S, Fukase H, Shimada J. Pharmacokinetics, safety, and tolerability of cefiderocol, a novel siderophore cephalosporin for Gram-negative bacteria, in healthy subjects. Antimicrob Agents Chemother. 2018;62(3): e02163–17.
Miyazaki S, Katsube T, Shen H, Tomek C, Narukawa Y. Metabolism, excretion, and pharmacokinetics of [14 C]-cefiderocol (S-649266), a siderophore cephalosporin, in healthy subjects following intravenous administration. J Clin Pharmacol. 2019;59(7):958–67.
Sanabria C, Migoya E, Mason JW, et al. Effect of cefiderocol, a siderophore cephalosporin, on QT/QTc interval in healthy adult subjects. Clin Ther. 2019;41(9):1724-36.e4.
Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C. Cefiderocol, a siderophore cephalosporin for Gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol. 2017;57(5):584–91.
Wenzler E, Butler D, Tan X, Katsube T, Wajima T. Pharmacokinetics, pharmacodynamics, and dose optimization of cefiderocol during continuous renal replacement therapy. Clin Pharmacokinet. 2022;61(4):539–52.
Kawaguchi N, Katsube T, Echols R, Wajima T, Nicolau DP. Intrapulmonary pharmacokinetic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, in patients with pneumonia and healthy subjects. J Clin Pharmacol. 2022;62(5):670–80.
Portsmouth S, van Veenhuyzen D, Echols R, et al. Cefiderocol versus imipenem–cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18(12):1319–28.
Naas T, Pogue JM, Kaye K, et al. Efficacy of cefiderocol in patients with nosocomial pneumonia and complicated urinary tract infections caused by Enterobacterales and carbapenem-resistant Enterobacterales (CREs) from three randomised clinical trials. 32nd ECCMID 2022, 23–26 April 2022, Lisbon, Portugal, Poster 1391.
Paterson DL, Kinoshita M, Baba T, Echols R, Portsmouth S. Outcomes with cefiderocol treatment in patients with bacteraemia enrolled into prospective Phase 2 and Phase 3 randomised clinical studies. Infect Dis Ther. 2022;11(2):853–70.
Longshaw C, Roger E, Santerre Henriksen A, Baba T, Nguyen S, Yamano Y. Evidence for efficacy of cefiderocol against OXA-48-containing isolates from the APEKS-NP and CREDIBLE-CR trials. Antimicrob Agents Chemother. 2022;66(10):e0110022.
Falcone M, Tiseo G. Cefiderocol for the treatment of metallo-beta-lactamases producing Gram negative bacilli: lights and shadows from the literature. Clin Infect Dis. 2022;75:1085–7. https://doi.org/10.1093/cid/ciac082.
Lampejo T, Cherian BP, Tan MGM, Wareham DW. Cefiderocol in the treatment of systemic carbapenemase-producing multidrug-resistant Klebsiella pneumoniae infection. J Glob Antimicrob Resist. 2020;23:338–9.
Trecarichi EM, Quirino A, Scaglione V, IMAGES Group, et al. Successful treatment with cefiderocol for compassionate use in a critically ill patient with XDR Acinetobacter baumannii and KPC-producing Klebsiella pneumoniae: a case report. J Antimicrob Chemother. 2019;74(11):3399–401.
Siméon S, Dortet L, Bouchand F, et al. Compassionate use of cefiderocol to treat a case of prosthetic joint infection due to extensively drug-resistant Enterobacter hormaechei. Microorganisms. 2020;8(8):1236.
Alamarat ZI, Babic J, Tran TT, et al. Long-term compassionate use of cefiderocol to treat chronic osteomyelitis caused by extensively drug-resistant Pseudomonas aeruginosa and extended-spectrum-β-lactamase-producing Klebsiella pneumoniae in a pediatric patient. Antimicrob Agents Chemother. 2020;64(4): e01872–19.
Bleibtreu A, Dortet L, Bonnin RA, The Cefiderocol French Study Group OBO, et al. Susceptibility testing is key for the success of cefiderocol treatment: a retrospective cohort study. Microorganisms. 2021;9(2):282.
Contreras DA, Fitzwater SP, Nanayakkara DD, et al. Coinfections of two strains of NDM-1- and OXA-232-coproducing Klebsiella pneumoniae in a kidney transplant patient. Antimicrob Agents Chemother. 2020;64(4): e00948–19.
Colombo F, Waheed A, Panese S, et al. Treatment with cefiderocol in K. pneumoniae KPC nosocomial external ventricular drainage meningitis: a brief report. Infez Med. 2022;30(3):454–8.
Monari C, Spagnuolo F, Pisaturo M, et al. Bloodstream infection due to a VIM-metallo-β-lactamase-producing Klebsiella pneumoniae treated with cefiderocol in a preterm newborn. Infect Dis Ther. 2022. https://doi.org/10.1007/s40121-022-00735-4.
Ackley R, Roshdy D, Meredith J, et al. Meropenem–vaborbactam versus ceftazidime–avibactam for treatment of carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2020;64(5): e02313–19.
Doi Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–75.
Doremus C, Marcella SW, Cai B, Echols RM. Utilization of colistin versus β-lactam and β-lactamase inhibitor agents in relation to acute kidney injury in patients with severe Gram-negative infections. Infect Dis Ther. 2022;11(1):187–99.
Rodrigues D, Baldissera GS, Mathos D, Sartori A, Zavascki AP, Rigatto MH. Amikacin for the treatment of carbapenem-resistant Klebsiella pneumoniae infections: clinical efficacy and toxicity. Braz J Microbiol. 2021;52(4):1913–9.
Ruiz J, Ramirez P, Company MJ, et al. Impact of amikacin pharmacokinetic/pharmacodynamic index on treatment response in critically ill patients. J Glob Antimicrob Resist. 2018;12:90–5.
Tamma PD, Bergman Y, Jacobs EB, Centers for Disease Control and Prevention Epicenters Program, et al. Comparing the activity of novel antibiotic agents against carbapenem-resistant Enterobacterales clinical isolates. Infect Control Hosp Epidemiol. 2022. https://doi.org/10.1017/ice.2022.161.
Banerjee R, Humphries R. Clinical and laboratory considerations for the rapid detection of carbapenem-resistant Enterobacteriaceae. Virulence. 2017;8(4):427–39.
Bradley N, Lee Y. Practical implications of new antibiotic agents for the treatment of carbapenem-resistant Enterobacteriaceae. Microbiol Insights. 2019;12:1178636119840367.
Emeraud C, Escaut L, Boucly A, et al. Aztreonam plus clavulanate, tazobactam, or avibactam for treatment of infections caused by metallo-β-lactamase-producing Gram-negative bacteria. Antimicrob Agents Chemother. 2019;63(5): e00010–19.
NCT03329092. A study to determine the efficacy, safety and tolerability of aztreonam–avibactam (ATM-AVI) ± metronidazole (MTZ) versus meropenem (MER) ± colistin (COL) for the treatment of serious infections due to Gram negative bacteria. (REVISIT). https://clinicaltrials.gov/ct2/show/NCT03329092. Accessed 2 Aug 2022.
Acknowledgements
Funding
The rapid service fee for this manuscript was funded by Shionogi & Co., Ltd., Osaka, Japan.
Medical Writing and Editorial Assistance
Editorial and medical writing support was provided by Adrienn Kis, PhD, and Joanne Shrewsbury-Gee, PhD, Highfield, Oxford, UK, and this support was funded by Shionogi & Co., Ltd., Osaka, Japan. Shionogi reviewed this manuscript from medical, legal, and regulatory perspective.
Authorship
All authors met the ICMJE authorship criteria.
Author Contribution
All authors contributed to the concept of the review and the literature search. All authors contributed to preparation of the first and subsequent drafts and reviewed each version. All authors approved the final version for submission.
Disclosures
Keith S Kaye has received consultancy fee from Shionogi, Merck, Qpex, Allecra, GSK, Spero. Thierry Naas has received non-financial support from Pfizer and personal fees and non-financial support from Shionogi, outside of the submitted work. Jason M Pogue has received consultancy fee from Shionogi, Merck, Qpex, Melinta, GSK, Abbvie, and Spero, and grant support from Merck. Gian Maria Rossolini has received consultancy fee from Menarini, Merck, Pfizer, and Zambon, he has participated in speaker’s bureau for Angelini, Menarini, Merck, Pfizer, and Shionogi. Gian Maria Rossolini has also received grant support for laboratory work from Angelini, Menarini, Merck, Nordic Pharma, Shionogi, VenatorX and Zambon, all outside of the submitted work.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kaye, K.S., Naas, T., Pogue, J.M. et al. Cefiderocol, a Siderophore Cephalosporin, as a Treatment Option for Infections Caused by Carbapenem-Resistant Enterobacterales. Infect Dis Ther 12, 777–806 (2023). https://doi.org/10.1007/s40121-023-00773-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00773-6